Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Broadband vibrational sum-frequency generation (SFG) is a second-order optical process that involves frequency mixing of three photons. Specifically, an broadband infrared (IR) and a narrowband visible (VIS) beams are overlapped in time and space at the interface, where they generate a third beam with the sum-frequency (ωSF=ωIR+ωVIS) of the two impinging beams. The electric field of the induced sum-frequency (SF) polarization P(2) (ωSF) is proportional to the effective second-order polarization of the interface: P(2) (ωSF)∝χeff(2) EIR EVIS. At interfaces of materials with inversion symmetry, the SF signal arises from both purely surface and contributions which are caused by the static electric field EDC in the electric double layer and can originate from adsorbed molecules that carry a net charge (surfactants1, proteins2, polyelectrolytes3 etc.), while other signals from the bulk solution can be neglected due to symmetry reasons. The effective second-order electric susceptibility χeff(2) of a charged interface can be expressed by:1
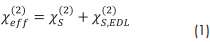
Here, the χS,EDL(2) term accounts for the probing depth and the phase mismatch ∆kz of the fundamental and SF waves at charged interfaces (EDC ≠ 0) that reflects possible interference effects at low ionic strengths.1 The pure surface χS(2) contribution can be written as a coherent overlap of vibrational bands that can contribute to the SFG spectrum. Here, we want to restrict the discussion only to homogeneously broadened bands with Lorentzian line shapes:
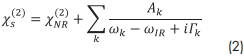
The oscillator strength Ak=N∫f(Ω)βk (Ω)dΩ, which represented by the amplitude of a vibrational mode k, depends on the number density N of interface-adsorbed molecules, their orientational distribution f(Ω) and on their hyperpolarizability βk.χNR(2) is a non-resonant contribution to the second-order electric susceptibility
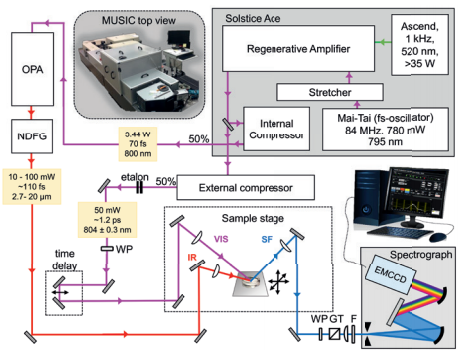
Figure 1 Schematic overview of MUSIC - Münster Ultra-fast Spectrometer for Interfacial Chemistry. Inset: Photograph of MUSIC showing a top view with the Andor Newton EMCCD detector and Kymera spectrograph in the front. Boxes are used to reduce stray light and to purge the IR beam path with dry air. Key: IR: broadband infrared pulse, VIS: narrowband visible pulse, SF: sum-frequency pulse generated, WP: retarders, GT: Glan-Taylor polarizer, F: short-pass filter, EMCCD: electron multiplying charge-coupled device.1
The Münster Ultra-fast Spectrometer for Interfacial Chemistry (MUSIC) is a user-friendly broadband sum-frequency spectrometer. MUSIC is composed of a 1 kHz Ti:Sapphire chirped pulse amplifier which is seeded by a Ti:Sapphire fs-oscillator. The amplifier delivers approximately 7 W (1 kHz, at 796 nm) average power. The uncompressed beam is divided by an internal (50:50) beam splitter. One beam is subsequently guided into an internal and the second beam to an external compressor. A total power of 3.2 W of the amplified and compressed fs beam (796 nm, 18 nm bandwidth) pumps an optical parametric amplifier with a subsequently unit for non-collinear difference frequency generation (NDFG) of the OPAs’ idler and signal photons. The NDFG unit generates broadband femtosecond IR pulses which are tuneable from 2.5 to 20 µm. The broadband IR has >300 cm-1 full width at half-maximum bandwidth. An air-spaced etalon (FSR 12.4 nm at 735 nm, R=94.5 %) was inserted into the external compressor to generate the VIS narrowband pulse centered at 804.1 nm and with a bandwidth of ~4 cm-1 bandwidth. Etalon side bands are removed by beam blocks, which are also placed inside the external compressor.
For the generation of the SFG, the VIS and the IR pulses overlap in time and space at 55° and 60° incident angles, respectively. For SFG spectroscopy, the SF beam is guided into a spectrograph (Kymera-328i-D2-SIL, Andor) where it gets spectrally dispersed using either a 1200 l/mm or a 1800 l/mm grating and imaged onto an electron multiplying charge-coupled device (EMCCD) (Newton DU970P-BVF, Andor). Prior to the spectrograph, a short pass filter with cut-off at 763 nm was used to filter unwanted photons.
For the liquid-gas interfaces, all spectra (Figure 2) were recorded using s-polarized SF, s-polarized visible and p-polarized IR beams, that is in the ssp polarization configuration. The polarization optics used are a half-wave plate for the VIS and a combination of an achromatic half-wave plate with a Glan polarizing prism of calcite. The Glan polarizing prims matches the polarization of the spectrograph-grating being then sensitivity-independent of the selected SFG polarization during the experiment.
In Figure 2, we show normalized SFG spectra of surfactant modified air-water interfaces that were obtained with the previously described spectrometer. For that the frequency of the tunable broadband IR beam was changed in 4 steps and SFG spectra were acquired, saved and stitched together afterwards. For that we have used a LabVIEW software that controlled both the OPA and with that the IR wavelength as well as the Andor spectrograph and EMCCD.
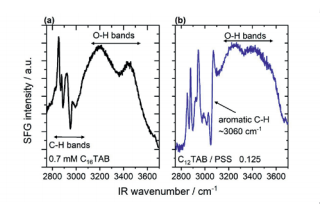
Figure 2 Vibrational SFG spectra of surfactant modified air-water interfaces from aqueous solutions with (a) 0.7 mM cetyltrimethyl ammonium bromide (C16TAB) and (b) dodecyltrimethylammonium bromide (C12TAB) mixtures with sodium polystyrene sulfonate (PSS) at a molar mixing ratio of 0.125 with respect to the monomer concentration of PSS:
[1] N. García Rey, E. Weißenborn, F. Schulze-Zachau, G. Gochev, B. Braunschweig, Quantifying DoubleLayer Potentials at Liquid-Gas Interfaces from Vibrational Sum-Frequency Generation, J. Phys. Chem C, 123, 1279-1286 (2019)
[2] M. Richert. N. García Rey, B. Braunschweig, Charge Controlled Surface Properties of Native and Fluorophore Labeled Bovine Serum Albumin at the Air-Water Interface, J. Phys. Chem. B 122, 10377-10383 (2018)
[3] F. Schulze-Zachau, S. Bachmann, B. Braunschweig, Effects of Ca2+ Ion Condensation on the Molecular Structure of Polystyrene Sulfonate at Air-Water Interfaces, Langmuir 34, 11714-11722 (2018)
[1] F. Schulze-Zachau and B. Braunschweig, CnTAB / Polystyrene Sulfonate Mixtures at Air-Water Interfaces: Effects of Alkyl Chain Length on Surface Activity and Charging State, Phys. Chem. Chem
Date: October 2020
Author: Prof. Dr. Björn Braunschweig, Center for Soft Nanoscience, Institute of Physical Chemistry, Westfälische Wilhelms-Universität Münster
Category: Application Note
