Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Light, or Visible Light, commonly refers to electromagnetic radiation that can be detected by the human eye. The entire electromagnetic spectrum is extremely broad, ranging from low energy radio waves with wavelengths that are measured in meters, to high energy gamma rays with wavelengths that are less than 1 x 10-11 meters. Electromagnetic radiation, as the name suggests, describes fluctuations of electric and magnetic fields, transporting energy at the Speed of Light (which is ~ 300,000 km/sec through a vacuum). Light can also be described in terms of a stream of photons, massless packets of energy, each travelling with wavelike properties at the speed of light. A photon is the smallest quantity (quantum) of energy which can be transported, and it was the realization that light travelled in discrete quanta that was the origin of Quantum Theory.
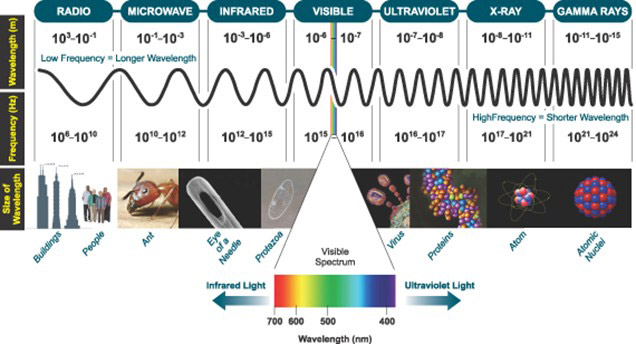
Figure 1: The Electromagnetic Spectrum, highlighting the narrow window of Visible Light that is detectable by the human eye.
Visible light is not inherently different from the other parts of the electromagnetic spectrum, with the exception that the human eye can detect visible waves. This in fact corresponds to only a very narrow window of the electromagnetic spectrum, ranging from about 400nm for violet light through to 700nm for red light. Radiation lower than 400nm is referred to as Ultra-Violet (UV) and radiation longer than 700nm is referred to as Infra-Red (IR), neither of which can be detected by the human eye. However, advanced scientific detectors, such as those manufactured by Andor, can be used to detect and measure photons across a much broader range of the electromagnetic spectrum, and also down to much lower quantities of photons (i.e. much weaker light levels) than the eye can detect.
It is no accident that humans can ‘see’ light. Light is our primary means of perceiving the world around us. Indeed, in a scientific context, the detection of light is a very powerful tool for probing the universe around us. As light interacts with matter it can be become altered, and by studying light that has originated or interacted with matter, many of the properties of that matter can be determined. It is through the study of light that, for example, we can understand the composition of stars and galaxies that are many light years away or watch in real time the microscopic physiological processes that occur within living cells.
Matter is composed of atoms, ions or molecules and it is through their interactions with light which gives rise to the various phenomena which can help us understand the nature of matter. The atoms, ions or molecules have defined energy levels, usually associated with energy levels that electrons in the matter can hold. Light can sometimes be generated by matter, or more commonly, a photon of light can interact with the energy levels in a number of ways.
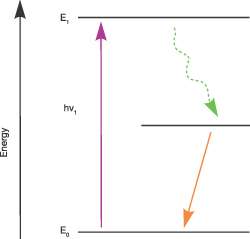
Figure 2 – Jablonski Diagram example, illustrating transitions between the various energy states of molecules following interaction with a photon.
We can represent the energy levels of matter in a scheme known as a Jablonski diagram, represented in Figure 2. An atom or molecule in the lowest energy state possible, known as the ground state, can absorb a photon which will allow the atom or molecule to be raised to a higher energy level state, known as an excited state. Hence the matter can absorb light of characteristic wavelengths. The atom or molecule typically stays in in an excited state only for a very short time and it relaxes back to the ground state by a number of mechanisms. In the example shown, the excited atom or molecule initially loses energy, not by emitting a photon, but instead it relaxes to the lower energy intermediate state by internal processes which typically heat up the matter. The intermediate energy level then relaxes to the ground state by the emission of a photon of lower energy (longer wavelength) than the photon that was initially absorbed.
Since photons that are either absorbed or emitted by matter will be of a characteristic energy, when the light that has interacted with matter is subsequently split into its constituent wavelengths using a spectrograph, the resulting spectral signature tells us a huge amount about the matter itself. The broad field of spectroscopy constitutes a multitude of spectroscopic techniques, such as raman spectroscopy, absorption/transmission/reflection spectroscopies, atomic spectroscopy, laser induced breakdown spectroscopy(LIBS) and transient absorption spectroscopy, providing a wealth of useful information on the scientific properties of atoms and molecules, as well as being able to very specifically identify the presence and quantify the amount of such materials in a sample.
