Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Model organisms, organoids and thicker tissues can be used to provide a more effective and complete means to study complex cell processes than is possible from cell line studies. However, such studies also bring with them many additional challenges. These challenges include the many aspects of how to optimize the optical set-up for effective imaging, through to handling the large data-sets these studies create. One of the fundamental considerations for any fluorescence microscopy measurements, and one that is compounded for imaging relatively thick living tissues, is autofluorescence – i.e. the natural emission of light by certain biological molecules [1]. The other key issue is that even though cells are largely transparent, as the light passes through the sample, components within the cells and tissues will serve to absorb and “scatter” the light and this is proportional to the depth of tissue being imaged.
Autofluorescence acts to interfere and obscure the detection of the fluorescence labels used within the experiment and manifests as a high background signal that reduces image contrast and clarity. With thicker samples autofluorescence will become worse due to there being a larger volume of auto-fluorescing tissue in the light-path. The spectra of autofluorescence is generally broad in nature and will occur across the visible spectral range. Unfortunately, this means that it overlaps with the emission wavelengths of most common fluorescent labels that are within the visible spectrum. Other sources of autofluorescence include cellular metabolites and components of culture media. Media can be intensely auto fluorescent, so care must be taken to reduce these sources to a minimum where it is possible to do so.
While Scattering of light can be used in a number of ways to infer information from a sample, scattering of light within a normal imaging context is deleterious to image quality. Scattering will affect both the excitation and emission wavelengths and reduce signal intensity and reduce the resolution of features within the image. Scattering is caused by changes in the refraction index within cellular components. Tissue clearing protocols may be used for some studies to create a more uniform refractive index throughout the sample. Absorption of light in the sample will increase as the depth of the sample increases. How significant this will be, will be specific to the experimental conditions, such as the level of highly absorbing substances like melanin or hemoglobin, the labels used, the imaging technique, the illumination levels that can be used without phototoxicity or photobleaching and the sensitivity of the detector.
Using NIR imaging approaches for study of thicker samples
An effective way to tackle the effects of autofluorescence and absorption and scattering is by using near-infrared (NIR) wavelengths above 700 nm. Above 700 nm auto fluorescence emission is dramatically reduced, as are scattering and absorption. There are also other practical benefits to being able to use the NIR region including a possibility to use more image channels.
Benefits of NIR imaging include:
Taking advantage of NIR imaging is of course not always quite so simple as all the supporting components need to be present. In recent years NIR fluorescent labels (probes) that absorb light within the 730-800 nm region and emit fluorescence around 750-1000 nm are now available [2]. Many standard fluorescence microscopes are not designed to operate at NIR wavelengths. For example, optics may include AR coatings which may reduce transmission of NIR wavelengths, or not be corrected for these wavelengths. Light sources such as the Xenon, metal-halide or gas discharge arc lamps used in regular widefield epifluorescence microscopes can have very low output in the NIR (typically they use heat-blocking IR filters). It is now possible to get illumination sources that are designed for NIR range – lasers are readily available, and more recently LED options. Modern sCMOS and EMCCD cameras would also be a requirement since older models may have low quantum efficiency and poor response to NIR wavelengths.
The Andor Dragonfly is a modern multi-modal confocal imaging system that has been designed to make NIR imaging possible. Key to this is the use of Borealis, with its Perfect Illumination Delivery™ technology that enables illumination of microscopic specimens with wavelengths that extend beyond the visible from the ultraviolet (UV) and through to the NIR regions.
The effectiveness in the NIR provided by Borealis illumination is demonstrated in the corresponding figure where the entire 90 mm thickness of an aged rat brain slice containing oligodendrocytes labeled with LiCor®IRDye 800CW NHS Ester has been imaged in 3D with 730 nm excitation and >785 nm emission. Tissues like these accumulate autofluorescent lipofuscin pigments that create a high image background when excited and imaged at more common visible wavelengths. All images were acquired with the same microscope hardware components (60x 1.4 NA oil immersion objective, iXon EMCCD camera).
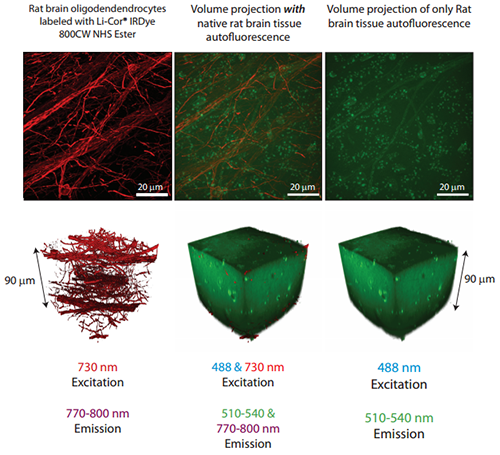
90 mm thickness of an aged rat brain slice containing oligodendrocytes labeled with LiCor®IRDye 800CW NHS Ester has been imaged in 3D with 730 nm excitation and >785 nm emission.
References:
