Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
With a range of high sensitivity detectors available, one of the areas where there is the most debate on the most suitable detector, is most certainly for single-molecule studies. EMCCD has established itself as the detector of choice for many single-molecule studies due to their unique ability to provide single-photon sensitivity. With the latest back-illuminated sCMOS offering a boost in QE to a comparable level as EMCCDs as well as the high speeds and fields-of-view that sCMOS is known for, is this now enough for back-illuminated sCMOS cameras to replace EMCCD cameras for single-molecule applications?
In this article Andor Product Specialists Dr. Justin Cooper and Dr. Alan Mullan discuss some of the most common questions they get asked on the different camera technologies and how this affects their suitability for single-molecule studies.
“In single-molecule studies one is imaging the light emitted from isolated, single fluorescent molecules. These molecules can either be individual small molecules, or fluorophores that are used to label larger macro molecules such as proteins or DNA, or even larger intracellular structures. These molecules can then be localized and tracked over time which can yield information on spatio-temporal distribution of the molecules of interest and on other phenomena that can induce a change in the location or fluorescence intensity of molecules such as chemical reactions, mass transport, bio-recognition, etc. Furthermore, studying phenomena at the level of single-molecule events allows one to probe the heterogeneity inherent in the system that would otherwise be lost due to ensemble averaging of bulk methods.”-JC
“Single-molecule techniques have found a wide variety of applications from material science where single-molecules have been used to probe the chemistry of surfaces at molecular distance scales, to biophysics where they have been applied to the study of the thermodynamics and kinetics of DNA hybridization, or mechanisms of immunological response. One of the most important applications of single-molecule techniques have been in their application to localization based super-resolution imaging.”-AM
“Some of the most common techniques that we would see being used in this research field would be Total-Internal-Reflection Fluorescence (TIRF) microscopy and light-sheet microscopy, which are used as methods of background reduction from light outside the depth-of-field of the microscopy system. Common measurement techniques that operate in the single-molecule regime are Fluorescence-Correlation Spectroscopy (FCS) which can be used to measure the diffusion of small populations of molecules, Forester-Resonance Energy Transfer (FRET) used as “molecular rulers” to measure distances scales at 10s of nm. Localization based super-resolution techniques like FIONA, PALM, STORM, DNA PAINT and combinations of these techniques are also a large application space for single-molecule imaging. The main aim of these approaches is to provide information on the underlying cellular processes that are otherwise hidden below the standard diffraction limit, as well as due to out of focus light within the illumination path. This makes it possible to look at the localization or dynamics of protein to DNA during replication or binding of a labelled protein to a specific receptor on a cell membrane. Studies using FRET or Fluorescence Lifetime Imaging (FLIM) enable you to look at the interactions of biomolecules over time with resolution ≤ 10 nm. FCS is another very useful technique that looks to be becoming a more common tool in the scientist’s toolbox as it can give data on things such as diffusion rates” –AM
View the most popular microscopy techniques article here.
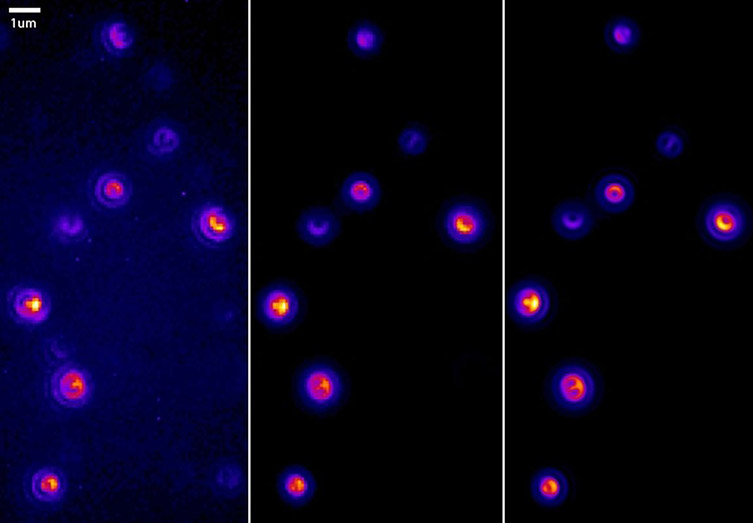
“I think the most important parameter is sensitivity. Single-molecule research applications can be by their very nature photon limited as single molecules inherently produce low numbers of photons compared with bulk measurements. Depending on the specific fluorophore being used and the time resolution requirements of the measurement, single-molecule techniques can quickly find their way into light levels of single-photons per acquisition. The fluorescent probes/dyes are also very susceptible to photobleaching. The camera therefore must be sensitive enough to detect very low numbers of photons per pixel - this could be in the order of 10 photons per pixel or less. Detectors have their own noise floor in the form of read noise and dark noise. If the noise floor of the camera is too high, then we cannot detect the image information we need. EMCCD cameras such as the iXon Ultra are capable of single photon sensitivity and effectively have negligible read noise. Back-illuminated sCMOS cameras typically have ~1.5-1.8 e- read noise. By using EM gain we can bring the noise floor down to a negligible level increase the signal-to-noise ratio.” -JC (This is shown in the image below).

Above: Increasing EM gain of the iXon EMCCD camera brings the signal of single molecules into view and improves the signal to noise ratio dramatically.
“Yes, this is the second most important parameter after sensitivity, because the image of single molecules, called the point spread function, needs to be adequately sampled to get the full spatial resolution of the optical imaging system. Applying Nyquist sampling theory, and using a typical high NA objective, would mean that for a 100x objective a pixel size of ~11 µm is ideal. This coincidentally is the size of the Sona back-illuminated sCMOS pixels. For 60x, 6.5µm would be optimal. The iXon Ultra 888 has a 13µm pixel which is optimal for 120x. It is common however to use additional magnification, sometimes called lens couplers, to adjust the sampling to suit the application. In other applications sometimes pixel size and oversampling are not quite as important but given the nature of single-molecule studies and its heavy use in localization techniques it would tend to be of high importance.” - AM
“Both EMCCD and back-illuminated sCMOS cameras do state up to 95% QE. This means that there is a 95% probability of a photon being absorbed and converted into an electron in the silicon sensor. At ultra-low signal levels, even a 95% QE is not enough to produce enough signal to overcome the read noise. The primary factor that makes EMCCD technology more sensitive is the electron multiplying gain register in the EMCCD camera. A single photoelectron will be amplified many times through a series of readout register pixels in a process called impact ionization. The result of this is that the low-level signal is amplified significantly (up to 1000x). By switching EM gain on you can now see low level information that would otherwise be hidden below the read noise of the camera.” -AM
Note: There is a full description of EMCCD technology in the Andor Learning Centre.
“EM gain noise – sometimes called multiplicative noise factor, is a noise that is due to the EM gain amplification itself. As electrons are passed from each pixel within the gain register there is a very small probability that additional electrons may be produced. Since this is executed many times during the EM gain process an additional variation to the signal. Effectively what this does is to increase the shot noise of the signal by a factor of Ö2 (1.41). The shot noise is the intrinsic variation of the signal itself and comes from Poisson counting statistics – which cannot be reduced for a specific photon flux. The EM noise should be considered an additional component of this. Because the shot noise is proportional to the square root of the number of photons in the signal, EM noise only becomes a significant component of the overall noise at higher signal levels and this is why the sCMOS cameras tend to have better signal-to-noise ratios at higher light intensities. However, in single-molecule experiments EM noise is a much smaller noise component given the ultra-low light levels that are involved, resulting in higher signal-to-noise ratios of EMCCDs at single photon light levels.”- JC
“Not necessarily - It is not just the frame rate that the camera can run at. In order to detect enough photons, the exposure needs to be set accordingly. Due to their amplification ability, EMCCD cameras can often use a shorter exposure time meaning the effective frame rate can be higher and you can get better temporal resolution. Furthermore, when cropped appropriately, EMCCDs can approach the frame rates of back-illuminated sCMOS sensors while still maintaining the ability to amplify the signal from these short exposures.”- AM
“If a wider field of view is required back-illuminated cameras like the Sona 4.2B-11 may be more suitable as with EMCCD you may have to reduce the region of interest to get the required frame rate. It really depends on the specific application.” -JC
“It has been interesting to see how this has settled out since the back-illuminated cameras have been introduced now for a few years, and many research groups have done comparative tests on these cameras. This is particularly important as they are dealing with the broad range of real-world applications, not just a smaller selection of research groups that we would be able to work closely with”. -AM
“Back-illuminated sCMOS cameras are now being used in single-molecule research. Their implementation has been aided by some developments like some new higher quantum yield fluorophores, but EMCCD cameras like the iXon Ultra series look set to maintain the strong foothold for some time due to their versatility with regards to single-molecule light levels. This contrasts with other areas of imaging where light is not so restricted, and we have seen back-illuminated sCMOS become the clear choice as well as the previous generation of front-illuminated sCMOS cameras like the Zyla 4.2PLUS which are great for general fluorescence microscopy.” -JC
“Some single-molecule research may not be within the lowest light regime that EMCCD is dominant – not every application is truly ultra-low light and there are now some bright synthetic probes. The back-illuminated sCMOS should provide a better signal to noise ratio after a certain cross over point, so for these less light-starved applications the back-illuminated sCMOS camera should be more suitable.” -AM
“There may also be applications that need a really wide field of view. In these conditions back-illuminated sCMOS detectors like the Sona 4.2B-11 are more likely to be a better option as you can cover more sample area in each image. Also, applications where time resolution is less of an issue and exposure times can be set long enough to have a larger signal level would be more amenable to the use of a back-illuminated sCMOS camera. Of course, no two applications are exactly the same, so it’s important to have a good understanding of the experimental conditions and where possible, demo the potential cameras on samples and experimental conditions that will be typical of your research.” -JC
“This is another factor which helps make EMCCDs suitable for many applications, especially where localization accuracy is important. The pixel-to-pixel variation is higher in sCMOS cameras due to the more complex readout process. This readout process does enable higher readout rates but there is a tradeoff in terms of pixel response uniformity (PRNU). In a sCMOS sensor each pixel has its own on-pixel amplifier so this means that each pixel will have a unique read noise component and each pixel will have a slightly different response to light. This is manifested as fixed pattern noise at the noise floor of the camera. For single-molecule experiments where signal levels are very close to the noise floor of the camera, the added pixel-to-pixel variation could affect both quantitative intensity information, as well as localization precision. The larger variation is somewhat mitigated by the unique pixel maps that are applied for sCMOS sensors which are used to correct for the variation. Cooling the sensor also lessens the impact of pixel noise like hot-pixels in sCMOS. However, CCD and EMCCD sensors have a simpler architecture and readout process and thus a better uniformity of intensity response, and essentially no fixed pattern noise. Thus, pixel-to-pixel variation will be a consideration for some studies, so it is important to be aware of this.”- JC
To conclude, both EMCCD and the new back-illuminated sCMOS cameras offer unique strengths. EMCCD cameras like the iXon EMCCD afford the highest sensitivity, with measurement of single photons. Whereas, back-illuminated sCMOS, like Sona, offer the combination of large field of view with fast frame rates coupled to sensitivity which is only slightly lower that of EMCCDs. The needs of each application must be considered to determine which camera would be the most suitable option. Given the broad range of experiments within the single-molecule field, it would be advisable to demo cameras of both technologies on both demanding and routine samples to see which one most suits your experimental needs.
Date: June 2019
Author: Dr Alan Mullan and Dr Justin Cooper
Category: Application Note
