Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Correlative Light and Electron Microscopy (CLEM) combines the two powerful techniques of light and electron microscopy to provide complementary information on biological samples across a wide size regime. Light microscopy allows for information to be obtained on fluorescently labelled live or fixed cells. Image resolution is limited to the approximately half the wavelength of light used to illuminate the sample; typically allowing features at a 250 nm size limit to be resolved. In light microscopy this diffraction limit can be surpassed using super-resolution techniques, which can resolve features down as low as 50 nm. Electron microcopy (EM) affords further improved resolution when compared with traditional light microscopy techniques, including super resolution techniques. Importantly, EM can resolve species at the molecular level, giving additional information on biological molecules compared with light microscopy alone. However, EM techniques, cannot be performed on live cells and samples must be prepared to fix cells or biological systems in place prior to analysis using EM to obtain suitable contrast of cellular features.
Within Life Science CLEM is starting to be used more widely, most notably, to improve understanding in the fields of neuroscience and virology, to examine samples within multicellular to molecular size regimes. CLEM is a valuable technique within Life Science because it combines the resolution of structural detail with the flexible labelling of fluorescence-based microscopy.
| Abbreviation | Definition |
| EM | Electron Microscopy |
| CLEM | Correlative Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| Cryo-CLEM | Cryogenic-Correlative Light and Electron Microscopy |
| ET | Electron Tomography |
| LSM | Laser Scanning Microscopy |
| EPi | Epi-fluorescent microscopy |
| STORM | Stochastic Optical Reconstruction Microscopy |
| PALM | Photoactivated Localization Microscopy |
| STED | Stimulated Emission Depletion |
| SIM | Structure Illumination Microscopy |
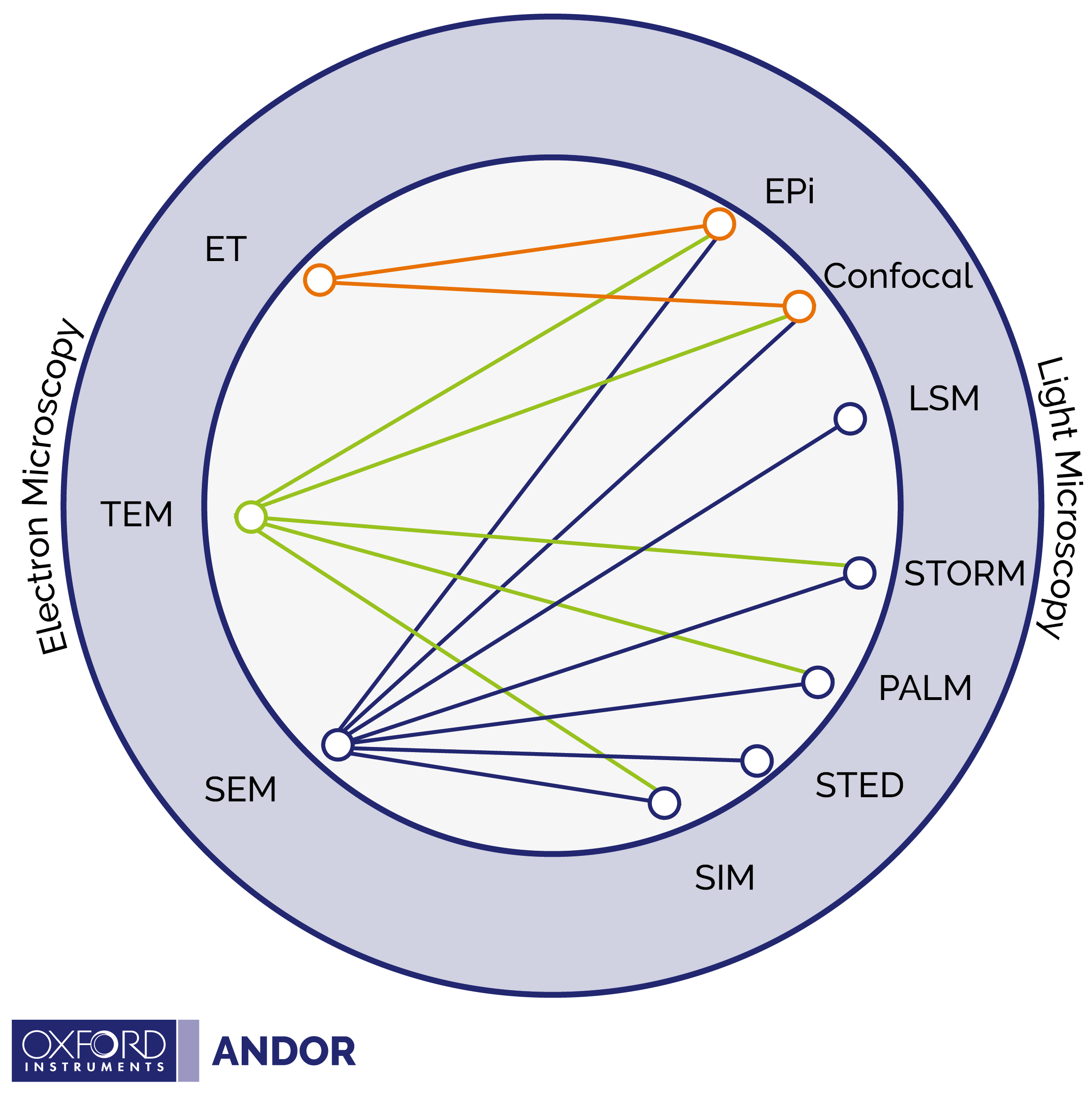
Typically, the electron microscopy portion of CLEM relies on Transmission Electron Microscopy (TEM) or Scanning Electron Microscopy (SEM). However, light microscopy analysis can be implemented using a number of different light microscopy techniques. This results in a large possible number of complementary combinations of light and electron microscopy techniques, which can make the field of CLEM experimental methods difficult to navigate. Please see our diagram below, adapted from Begemann and Galic2 which presents a simple graphic illustrating which combinations of light and electron microscopy techniques have been successfully implemented. Definitions of the abbreviations are provided in the table above.
For both light and electron microscopy the method of sample preparation is dependent on the specific experimental technique used. Light microscopy relies on fluorescent labelling of biological species; and there are a wide range of fluorophores available. In contrast, electron microscopy is dependent on whether TEM or SEM is used. TEM involves sample dehydration and sectioning into small slices (using a ultramicrotome), whereas prior to analysis in SEM the sample is coated with metal.
Electron microscopy techniques focus on a smaller XY area in the sample when compared with an image taken using light microscopy and directly comparing the areas measured using the different techniques can be challenging. As a result, integrated CLEM systems often come in two variants, the first with a fixed field of view for both light and electron microscopy measurements and second where the viewing area can be moved when switching between imaging techniques. Typically, biological, or artificial markers can be used as a reference point to indicate the area of interest and ensure the same area is examined using the two complementary techniques.
This article is intended as an introduction to the variety of CLEM techniques available, technical reviews are linked within the references section for further reading.
