Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Fluorescence microscopy, aided most notably by the development of labels such as GFP and its variants, have enabled significant advancements in our understanding of the many processes within cells, and various model organisms [1]. While many different labelling strategies and types of fluorescent labels have been developed, the majority of labels emit within the UV-visible spectrum of 400-700nm. The optics, filters and the detectors of the imaging system have also been optimised around this window to ensure maximum transmission, and thus optimal detection of the emitted wavelengths.
Today’s researchers are building on existing studies by looking towards more biologically relevant tissues and use of models such as organoids and small animals, to gain greater understanding of processes within the whole organ or organism context. New challenges emerge as a result, one of which is imaging thicker tissues within the UV-visible spectrum. The wavelengths within the UV-visible window are not well suited to imaging of thicker samples since these wavelengths are attenuated as they pass through tissues by absorption and scattering. There is also autofluorescence of various biological compounds e.g. collagen, elastin, NADPH and cellular organelles such as mitochondria, and red blood cells. Shorter wavelengths towards the UV region are more phototoxic to the cell and disrupt normal cell function. Of course, there are various ways to mitigate these problems to some degree for some samples. Tissue clearing, is one such example- but this does not work for live-cell, or pin vivo models. We can use light-sheet, confocal imaging or multi-photon imaging to reduce out of focus signal, and adjust illumination power by sample depth to improve the contrast and thickness of sample we can work with to some extent. However, the problem of UV-visible wavelengths being attenuated and scattered in thicker samples remains.
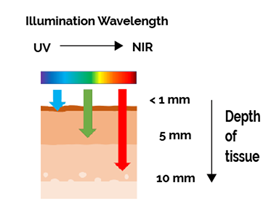
Figure 1: Longer wavelengths penetrate deeper into tissues making them more effective for imaging of thicker samples. Shorter wavelengths are also know for inducing greater damage to cell function e.g. DNA damage, organelle damage.
The solution therefore is to shift to using longer Near Infra-Red (NIR) wavelengths up to 900nm and beyond. The first NIR window, NIR-I, encompasses the 700- 950 nm range. By imaging within this region there is less scattering, and reduced absorption by tissues [2]. A wide range of labels have been developed e.g. Cy7, Alexfluor series and others. Autofluorescence of a number of components within some tissues remains – notably water with a strong peak around 900-1000nm. By using the NIR-I window it is possible to extend the effective imaging depths up to 1-2 cm which is useful for both imaging and spectroscopy-based studies.
For deeper imaging e.g. for small in vivo studies with mice, it is necessary to move higher into the NIR II (Near InfraRed II), also called the SWIR (Short Wave InfraRed) window. By using this part of the spectrum, we can greatly reduce scattering of light and absorption and avoid some of the main autofluorescence wavelengths – including water present in the 900-1000nm range.
Broadly speaking applications that may benefit from use of NIR imaging are thicker samples and specimens where gentle imaging is needed to study development or processes over extended durations. Some examples include:
There are already a wide range of fluorescent labels available for the NIR I, and the range of NIR II inorganic probes and organic dyes is starting to grow. Exploiting the benefits of the NIR II window for imaging is therefore increasingly applicable to more researchers to consider. Some classes of NIR II labels are:
Each of these classes of labels offer benefits in terms of brightness, stability and biotoxicity and have found use in various preclinical studies. Indocyanine Green is a relatively well-known organic dye and has been clinically approved and used for some time. The emission is at the upper end of NIR I into the NIR II. The use of heavy metals, low quantum yields and low specificity are potential disadvantages of some of the other NIR II labels, but these are being superseded by brighter, more biocompatible and more target-specific variants [3,4,5,6].
One of the most important considerations in any imaging system is the choice of detector. The sCMOS and CCD detectors that are used for most general microscopy applications in the UV-Vis range are silicon based. The response of standard silicon detectors drops off after 700nm and is typically less than 50% after 800nm. This is due to longer wavelengths passing through the photosensitive layer of the sensor without forming a photoelectron. In fact, some of the latest back-illuminated sCMOS cameras have an even poorer performance towards, and within the NIR. Normal sCMOS and CCD sensors are therefore poorly suited for imaging within the NIR I region.
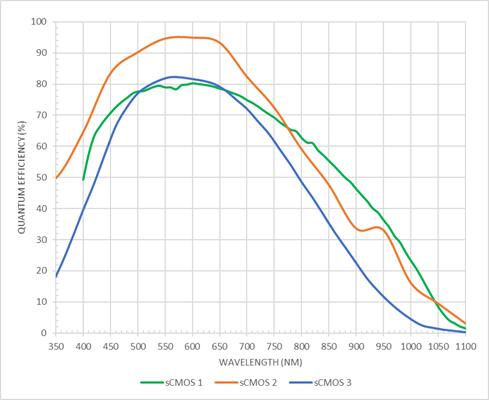
Figure 2: Modern microscopy cameras, such as the sCMOS examples shown here offer high QE of 80-95% centred around the UV-vis wavelengths. However, the response of these cameras starts to drop off above 600nm, and QE may be 20-40% towards 900nm making them poorly suited to NIR I based imaging.
A small number of CCD detectors such as the iKon-M deep cooled CCD do offer a “deep depleted” sensor option. These specialised sensors boost the sensitivity of the 700-900nm range by extending the depth of the photosensitive region of the Silicon. This increases the probably of incident photons generating a photoelectron and thus the sensitivity is greatly improved.
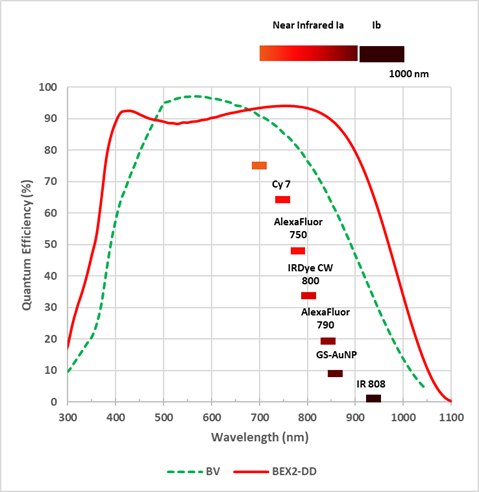
Figure 3: Even the broadest response normal CCD technology sensor options such as the “BV” suffers drop off towards the NIR I region. The Deep cooled CCD camera iKon-M features a BEX2-DD (deep depleted) sensor option that extends response through to 900nm.
A further specialisation of these sensors is an anti-fringing treatment that greatly reduces etaloning internally reflected waves that can create distortions when imaging at these longer wavelengths (etaloning). While the iKon-M camera with the deep-depleted CCD sensor offers good sensitivity in the NIR region, it is a CCD sensor meaning that is only suited to slow, long exposure applications such as bioluminescence and some limited fluorescence applications that do not require the speed typical of modern sCMOS sensors
When it comes to imaging in the NIR II region, a different detector technology is required than Silicon. InGaAs (Indium gallium arsenide) detectors have been available for many years [7], mostly for spectroscopy applications but there have been few solutions for high sensitivity imaging applications. A new generation of InGaAs sensors is now available that offers improved sensitivity, speed and field of view. These are therefore now a viable option for in vivo imaging applications such as tumour development and studies within organs such as cardiac function, neurobiology and small animal in vivo studies. Some of these imaging experiments require high speeds such as studying blood flow within vascular and cardiac tissues, whereas others require longer exposures- such as luminescence based experiments [8].
The new C-RED series from First Light Imaging features a number of InGaAs technology-based cameras (and a unique photon counting SWIR camera based on an e-APD MCT sensor) that are optimised for the NIR II window. Of these, the C-RED-2 is most suitable for life science imaging applications.

Figure 4: The C-RED-2 camera is based around a new generation InGaAs sensor that offers a broad response across the 900nm to 1700nm region. When combined with a 640x512 pixel array and high speeds up to 600fps it makes it suited to a range of applications that utilise the NIR II window.
C-RED 2 is a revolutionary ultra-high speed, low noise camera for demanding NIR II imaging applications. The C-RED 2 has a 640x512 array with 15 µm pixels that can provide a high resolution over a wide field of view. The quantum efficiency between 900-1700nm is greater than 70% meaning that sensitivity is high across the full NIR II window. A very high speed of 600 fps can be achieved, backed up by an electronic shutter is present with integration pulses shorter than 5 μs. This makes the C-RED 2 ideal for dynamic processes in tissues and small animal models including blood flow, cardiac and cell signalling. Read more about the C-RED-2 being used for NIR imaging of small animal models here: In vivo NIR-II Small Animal Imaging with C-RED 2
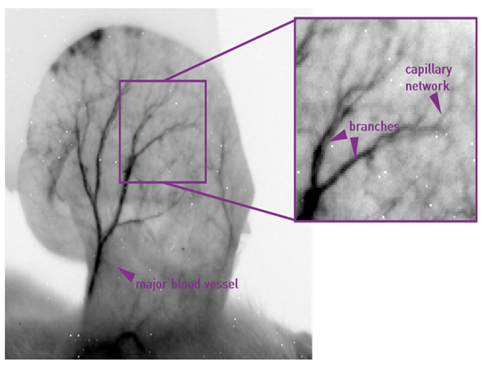
Figure 5: The fine structure of capillary networks can be observed in developing organs in small animal models.
C-RED 2 is also suitable for longer exposure applications with deep cooling as low as -40°C keeping the dark current low. This serves the needs of luminescence-based experiments that require much longer integration times, with cooling being a key requirement of InGaAs sensors to keep the inherently higher thermal noise as low as possible. You can find out more about the C-RED 2 is ideal for longer exposure applications here: Long Exposure Times - How to Optimize your Acquisition with C-RED 2

Figure 5: The C-RED 2 InGaAs camera from First Light Imaging is a new addition to the Oxford Instruments Andor Camera range.
The C-RED 2 features a C-mount making it easy to integrate with custom built imaging systems. Note that most standard microscope stands are designed with the UV-Vis and some NIR I imaging capabilities in mind, and will not be compatible with imaging in the NIR II region. However, given the nature of the stage of NIR II based imaging and the specimens that will typically imaged, most systems will need to be customised and optimised to exclude light, and optimise the NIR II wavelengths. You can read more about how the C-RED 2 has already been used to help develop more effective NIR II probes: Development of Fluorescent Biomarkers with C-RED 2- Oxford Instruments (oxinst.com)
Control of the C-RED 2 can be done using python, LabVIEW, via SDK, micro-manager or First Light Vision software.
References
Date: September 2024
Author: Alan Mullan
Category: Technical Article
