Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
In this article we discuss the key configuration factors impacting the price of confocal microscopy systems. Many factors affect the price of confocal microscopy systems and when purchasing a microscope, one must consider the complexity of the instrumentation and the capabilities it provides. Clearly the more complex the confocal, spinning disk, widefield or TIRF system configuration, the higher the associated cost of the system. Many choices are posed to the potential buyer: options for multiple imaging modes, highly sensitive detectors, simultaneous multicolour imaging, and multimodality.
In this article we give an overview of the underlying hardware of confocal microscopes and the possible choices available when deciding on a confocal microscope system suitable for your application. We will start by simply deconstructing a spinning disk confocal system and will briefly discuss systems with other imaging modalities such as Super-Resolution, and TIRF. In addition to hardware costs, the software impacts the overall cost of a confocal microscope, including what options are included in the basic software package, and the extended software options. Moreover, longer term maintenance and system servicing costs should also factor in when coming to a final decision on your choice of confocal microscope system.
Overall, we provide an extensive list of hardware, software and maintenance options that are poised for the buyer when deciding on a system suitable for your applications. Hopefully, we will provide some clarity for the reader on why confocal microscopy systems justify their cost.
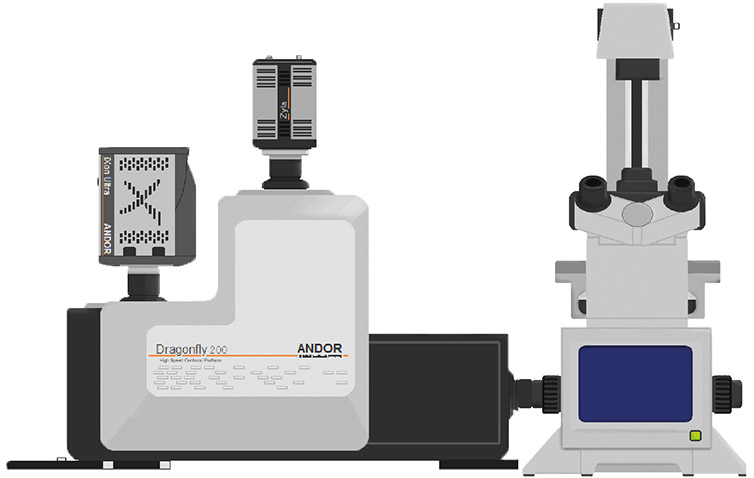
Figure 1- Schematic of Andor’s Dragonfly spinning disk system attached to an epifluorescence microscope.
Please use the table below to navigate to a section of interest on the components that impact on confocal microscope configuration and will influence costs.
Click below on the the key configuration factor to navigate and learn more about the implications on the cost of the microscope.
| Options | Comments |
| Single pinhole | The pinhole discards out focus light: 40 µm pinhole is ideal for high magnification (63X and 100X). 25 µm pinhole is indicated for lower magnifications. A dual pinhole microscope will offer more versatility but will be more expensive. |
| Dual pinhole |
Extra note: The choice of pinhole is relevant for spinning disk confocal since this type of equipment has fixed pinhole size(s). For point scanner confocal microscopes, the pinhole selection is not an issue when acquiring the equipment.
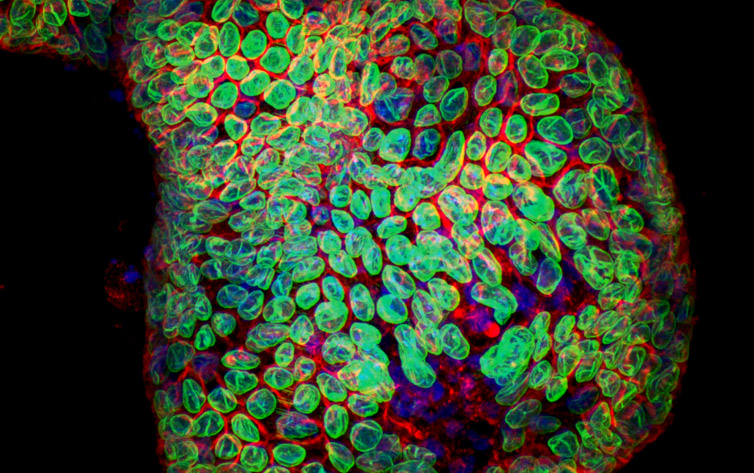
Figure 2 : Organoid image. Image acquired with Dragonfly spinning disk confocal system using 25 um pinhole. Image courtesy of Dr Luke Boulter, MRC Human Genetics Unit, IGMM, University of Edinburgh.
| Options | Comments |
| Yes/No? | Extra magnification in front of the camera port allows for acquisition under Nyquist Criteria for cameras in which the pixel size is greater than 6.5 µm. Larger pixel sizes gather more light, but will not meet Nyquist at lower magnifications. Extra magnification may can be achieved by a fixed or motorized lens (to allow switching). Acquiring under Nyquist or strict Nyquist criteria is an essential requirement for deconvolution and Super-resolution microscopy (see requirement 15). Different microscope companies offer different options of extra magnifications that can be used in combination with the camera to acquire under Nyquist conditions. |
| Options | Comments |
| Single/double | Dichroics can be single or multiple band pass. Multiple band pass dichroics allow simultaneous multicolour imaging. Band pass dichroics are useful if dual camera acquisition mode is used. The use of various dichroics is possible and delivers flexibility in acquisition. Motorized or manual dichroic switch is also an option. Dragonfly spinning disk system supports 4 dichroics, interchangeable via a motorized switch. |
| Triple/quad | |
| Band pass |
| Options | Comments |
| How many? | Multiple band pass emission filters will allow faster multicolour imaging, but if the filters and the fluorochromes used are not very accurately chosen, the resulting image might present bleed through between channels. Single band pass emission filters significantly minimise the possibility of bleed through. The number of emission filters chosen will directly influence the number channels that can be acquired in a multicolour imaging experiment. |
| Single band pass | |
| Multiple band pass |
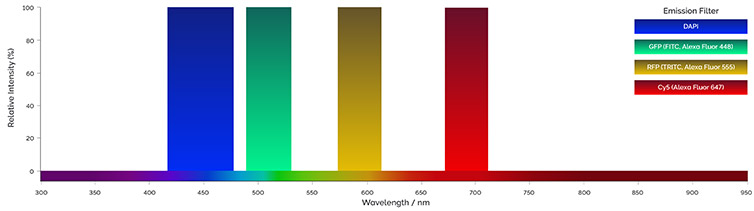
Figure 3 - Example of emission filters bandpass. Single or multiple band pass filters can be used to acquire multicolor images.
| Options | Comments |
| Multiple laser line source | How many lasers can the unit accommodate? 4 lasers, 8 lasers? What is the range of laser wavelengths supported by the laser Unit? What is the laser power supported? |
| Options | Comments |
| How many lasers? | The number of lasers as well as their wavelengths will directly impact the number of channels that are possible to acquire, as well as the experimental design. Using a laser unit that can accommodate Near Infra-Red (NIR) wavelengths (>650 nm), can provide experimental advantages. NIR lasers allow deeper sample penetration and since NIR lasers are less energetic they are useful for live imaging experiments. Single mode fibre provides an effective point source for diffraction limited point scanning. Nevertheless, single mode fibre will not efficiently couple or transmit longer wavelengths. Conversely, Dragonfly’s multimode optical fibre combined with the patented illumination system, Borealis™ can support wavelengths of excitation and detection in the ranges 400–800 and 425–850 nm respectively. Higher laser powers might be required for specific applications such as Super-Resolution (STORM) or laser ablation experiments. Specific experimental requirements will impact on the type, number, laser wavelengths and powers required. As such, the laser choices will directly affect system complexity and ultimately the cost. |
| Single mode or multimode fibber? | |
| What is the laser power required? |

Figure 4 – Integrated Laser Engine. Image shows Andor´s Integrated laser Engine, that supports up to 8 laser lines and accommodates visible to the NIR range lasers in a single unit.
| Options | Comments |
| Photomultiplier tubes (PMTs) & Hybrid detectors | Traditionally point scanners make use of photomultiplier tubes (PMT) and hybrid detectors, whereas multipoint confocals use camera-based detectors. But what is the main difference between PMT and sCMOS or EMCCD detector technology? There are several parameters that are used to evaluate the performance of a scientific detector. Important parameters to take in consideration are: Quantum Efficiency (QE), Dark Current, Read Noise, and Signal to Noise Ratio. Camera based detectors are much more sensitive and effective in capturing photons than PMT and hybrid detectors. The quantum efficiency of a PMT and hybrid detector is a maximum of 45% whereas the quantum efficiency of sCMOS and EMCCDs generally averages between 80 to over 95%. The dark current is also lower in EMCCDs and sCMOS detectors when compared to PMTs: EMCCDS (< (0.001 e-), sCMOS (< 1 e-) and PMTs (around 2 e-). When PMTs were introduced to the market they were the clear front runner detector as they have no read noise. Nevertheless, currently, with modern camera technologies, the Read Noise is very low for EMCCDs and sCMOS detectors; <1 e- effective read noise is possible for EMCCDs using “EM gain”, and around 1.1 to 1.6 e- for sCMOS models. Overall camera-based detectors (EMCCDs and sCMOS) deliver much greater sensitivity than PMTS and Hybrid detectors. |
| Cameras (sCMOS and EMCCDs) |
Extra Note:
Quantum Efficiency (QE) - the capacity of a detector to efficiently convert a photon to an electron. The higher the QE, the more sensitive the detector is (e.g 50% QE would mean half of the photons are converted to electrons)
Dark Current – Is the measure of the noise that generated by the sensor itself in the absence of any signal and expressed as electrons (e-)/pixel/sec.
The lower the value the better for any given equipment. Since it increases with time, it becomes more important as exposure times lengthen. Camera cooling can be used to reduce the dark current. Cooling may be in the form of air cooling, water cooling (see note below)
Read Noise - Read noise is created as the camera electronics convert the electrons from analog to a digital signal and the signal is amplified. The lower the value the better as this sets the noise floor of the camera and thus the ability to detect a signal of interest.
Cooling - As mentioned, cooling the camera sensor reduces dark current. Cooling may be passive (relying on exchange of heat from camera body to surrounding air), or air cooled using a fan to allow improved cooling. Water cooling provides the deepest possible cooling and is an option on some camera models. A further benefit of water cooling is that it eliminates the use of a fan that may act as a source of vibration. (Important for vibration sensitive measurements e.g. high frame rates at high magnification, or localisation microscopy, since vibration impacts the image data). Cooling also reduces the possibility of “hot” or high noise pixels that could impact the image especially in image stacks or 3D volumes.
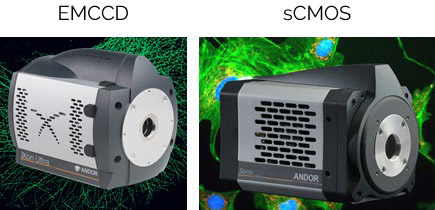
Figure 5 Image of iXon Ultra 888 EMCCD and Sona 4.2B-6 sCMOS.
| Options | Comments |
| sCMOS | sCMOS and EMCCDs have relative strengths and weaknesses for different experimental requirements. sCMOS sensors have a sensor architecture that allows faster speeds and image acquisition with larger fields of view, thereby increasing experimental throughput and productivity. Some sCMOS detectors have a 6.5 µm pixel size ideal for acquisition under Nyquist conditions typical magnifications; these cameras also allow for ultra-fast imaging. Therefore, sCMOS are very suitable for the most common imaging applications. EMCCDs have superior sensitivity and are still the best option for very faint signals or very low light imaging condition. Additionally, due to the very low dark current of deep cooled EMCCD cameras, they are more suitable for long exposure applications, e.g. luminescence studies. Currently, many spinning disk confocal systems can accommodate two cameras. If, when purchasing the system two cameras are not required, when possible one could choose a system that can later accommodate a second camera. By doing so, one will have a certain amount of freedom to accommodate future experimental needs. Having two cameras on the confocal system will allow more flexibility, speed, and simultaneous multicolour acquisition. Example experimental set up A system with an EMCCD and an sCMOS camera in which the sCMOS pixel size can be binned to match the EMCCD and the chip size matches between both cameras, will allow simultaneous double colour imaging. (E.g an EMCCD camera with a 13 µm pixel matches a 6.5 µm pixel of an sCMOS camera when 2x2 binning is used.) Moreover, the system will also deliver the concurrent benefit of large FOV and smaller pixel size for sCMOS and the advantage of very low light imaging with the EMCCD. Ultimately the number of cameras and their specifications will rightly impact on the overall cost of the microscopy system. Remember that improvements in camera technology allow for modular upgrades overtime; moving to the latest detector technology can significantly improve the system's imaging performance. |
| EMCCD | |
| Multiple cameras |
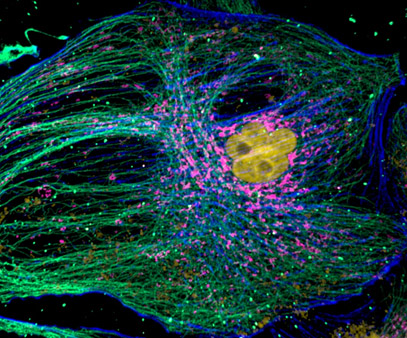
Figure 6 - mammalian cell cytoskeleton. Image acquired with Dragonfly spinning disk confocal microscope using an iXon 888 EMCCD camera. Image courtesy of Hao Hui Wen (Dr. Sun Yu Jie Lab), Peking University.
| Options | Comments |
| Brand | There are several options for the microscope stand; the first choice would be what brand to buy. Different brands offer different options. One crucial point to consider, and that is often disregarded, is how ergonomic it is; as users spend countless hours on the microscope when acquiring and assembling a system, the users' comfort should not be ignored. It is valuable to consider this aspect, as it could impact health and, ultimately, productivity. Important questions to consider include:
The first choice will be between an upright or an inverted stand. Choosing between upright or inverted will depend on the type of experiments to be performed. For example, electrophysiology experiments and intra-vital experiments such as live blood vessel imaging will most often require an upright microscope stand. In contrast, mammalian live-cell imaging is most often performed using an inverted stand. Users need to consider the nature of experiments to be performed and choose the type of microscope that is better suited to perform those experiments. Answer 2) How many ports does the microscope stand have? Is there a requirement for attaching other imaging/optical devices to the microscope? Is there a requirement for photostimulation devices? Is there a future requirement for adding other optical components to the system? Suppose the user plans to add (at time of purchase or in the future) other optical components to the microscope stand. In that case, when buying the microscope stand, a double-deck microscope should be chosen. By choosing a double-deck microscope stand (even if paying a little bit more when acquiring the equipment), the user will keep the options open for future applications. Using a double-deck microscope combined with a photostimulation device will deliver a better user experience for sample visualisation. Another possibility would be to attach a camera to allow additional imaging applications such as combining bioluminescence with fluorescence imaging. |
| Upright / Inverted |
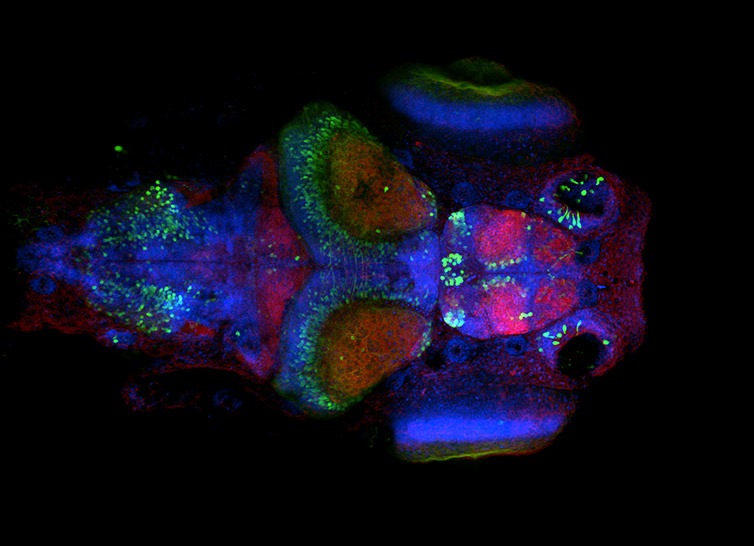
Figure 7 - Neural system in Zebrafish larvae. Image acquired with Dragonfly spinning disk microscope using an inverted microscope stand. Simultaneous acquisition with two Zyla sCMOS cameras, band pass dichroic 25 um pinhole, and single band pass emission filters. Image courtesy of Ruth Diez del Corral and Davide Accardi, Champalimaud Centre for the Unknown.
| Options | Comments |
| Manual /Motorized | Following choosing the stand, the microscope stage is a key factor. Does the stage need to be motorised? Since motorised stages will increase the device's price, what are the advantages of including a motorised stage? Motorised stages will increase productivity, allowing the acquisition of multiple tiles on XYZ with one click; it is a wise choice when possible. It also facilitates large area, multi-field, montage imaging for large samples and in-tact tissues. Other options related to stage choice and XYZ include a piezo-based stage. Again, Piezo controlled stages will increase the price but offer important advantages that might be required for certain experimental setups. Piezo stages offer benefits when it comes to very smooth movement with nanometre and sub-nanometer precision for 3D imaging. They also excel in step & settle times, as well as fast scanning. Therefore, a piezo controlled stage could be a good option if the users require fast acquisition speeds and high accuracy on the movement. |
| XYZ precision (Piezo) |
| Options | Comments |
| Magnification | An objective is an essential component of the microscope and should be chosen wisely for the required applications. Choosing objectives is on its own an enormous task due to the vast number of options to choose from at each magnification. Initially, users start to select how many objectives the microscope will have, and the desired magnifications. The Numerical Aperture is the ability of the objective to capture wider rays of light, a wider cone of light will deliver higher resolution power of the microscope. The main question at this point would be: what resolution is required? High-end applications such as dSTORM or Total Internal Reflection Microscopy (TIRF) will require objectives with extremely high NAs (>1,4). High NA objectives are costly. Another consideration is the working distance of the objective, this is an application specific requirement. For example, long working distance objectives are fundamental to:
The immersion media of an objective is also an important point. To maximise the collection of light rays and avoid diffraction, the immersion media of the objective should have a refractive index as close as possible to the immersion media of the sample. Commonly used immersion media are air, oil, water, silicon, and glycerol. Most often air objectives are cheaper and immersion objectives are more expensive. When choosing an objective for fluorescence imaging is essential to remember that not all objectives will transmit equally in all wavelengths. Experiments involving NIR and UV wavelengths should be given special attention, when considering the optics of your system. If one wants to image DAPI, it is essential to have an objective that transmits on the UV range. Conversely, when imaging NIR wavelengths, consideration must be taken to ensure that the objective transmits at these wavelengths. There are many aberrations caused by light passing through the glass that are corrected in different objectives; different corrections imply different prices. Objectives can be:
Plan means that the objective is corrected for Field Curvature. Field Curvature distortions impair the proper focus of an object on a flat plane and therefore severely impact the quality of the image. When the budget allows Field, curvature correction (Plan) Objectives is a critical choice. In Spherical Aberration, the rays of light of an objective lens suffer distortions in the edges (more curved), and they will not focus on the same plane as the rays of light that went through the center of the objective, causing an image that is blurred. Achromat objectives correct Spherical Aberration for 1 wavelength, Fluorite for 2-3, and Plan Apochromat for 3-4. Chromatic Aberration is the failure to focus all wavelengths on the same plane. Achromatic objectives offer correction for 2 wavelengths, Fluorite 2-3, and Plan-Apochromat 4-5 wavelengths. Finally, one must consider the objective supported techniques because not all objectives support all imaging modalities. Imaging modalities available are BF-brightfield, DF-Dark Field, Ph- Phase Contrast, DIC – Differential Interference Contrast, Pol – Polarisation, Fl – Fluorescence. Please remember than one objective can support multiple imaging modalities. Overall, the final cost of each objective will depend on a combination of all the parameters described above. Often money can be saved if choosing a microscope stand for which there are already available compatible objectives. (see requirement 9 – microscope stand). |
| Numerical aperture | |
| Working distance | |
| Immersion | |
| Transmittance | |
| Corrections |
Video 1 - Mouse Hippocampus Expanded sample captured with Andor Dragonfly rendered in Imaris. In the video the researchers took advantage of the large dragonfly FOV and of Borealis highly Uniform illumination. The result delivered a perfectly stitched Mouse hippocampus movie where the detail on the brain cell can be clearly visualized. Courtesy of Jennifer Santini, UCSD School of Medicine, Microscopy Core Facility
| Options | Comments |
| Brightfield | A transmitted light technique might be of interest to the user. One of its current uses is to overlay on the confocal fluorescent image providing an overview of cellular and tissue shape. Brightfield has less complex optics (and therefore cheaper) but many cells and tissues are nearly invisible through this technique. Phase contrast microscopy will increase the visibility of the transmitted light images, still many images will appear with a distractive phase halo. DIC (Differential Interference Contrast) is the transmitted light technique that provides the higher resolution and contrast. DIC optics are complex and more expensive than the options above. Several components need to be purchased considering the transmitted light technique that is chosen, e.g. phase plate rings, polarizers, and specific objectives to match the transmitted light technique to be used. |
| Phase contrast | |
| DIC (Differential Interference Contrast) |
| Options | Comments |
| Top stage incubator | If live imaging is a crucial application, then an incubation chamber is also an essential requirement, there are a multitude of options to choose from. When deciding take into consideration the applications, as well as the other choices for the system: the stage, microscope stand, microinjection devices, etc. The aim should be to make the confocal system a fully integrated system in which the choices of every component are suitable for the desired applications and function appropriately together. The user can opt for: 1) a stage top incubator, 2) a bottom stage incubator that would match different slides or Petri dishes used in the lab, or, 3) a large incubator to warm up all the system. The final option would be a combination of a large incubator to warm up all the system and a heated sample holder for higher accuracy. Key aspects to take into consideration are:
Overall, the more options you add to the incubator, the more expensive will be, as for such an incubator that will also allow CO2 control will be more expensive than an incubator which controls only the temperature. |
| Bottom stage incubator | |
| Large stage incubator |
Video 2 – In vivo imaging of blood flow in mouse intestine. Image acquired with with Andor Dragonfly at a 200 frames per second using Andor sCMOS camera. Live tissue was maintained at 37ºC with an incubator. Sample courtesy of Dr. Takahiro Kuchimaru. (Jichi Medical University, Japan).
| Options | Comments |
| Rigid | A rigid table provides no isolation against vibration, although if the construction is strong enough, they can deliver down to 1 µm resolution. The more sensitive the application, the more important it is to have a proper table for the system to stand on. To deliver the perfect image, a table should absorb the vibrations caused by the surrounding environment. Certain applications such has STORM, SRRF-stream and TIRF can only be executed using an anti-vibration table. An antivibration table is also extremely useful for applications such as time lapse imaging, large tile imaging, where matching the moving continuous frames, or acquired tiles is essential. Again, the price varies according to the table chosen, e.g., according to the level of vibration isolation that the table can provide. |
| Passive | |
| Active |
Note: As a general rule, the resolutions offered by tables are:
Please note that these numbers are indicative, and when purchasing an equipment ensure that the table you acquire provide the necessary vibration isolation for the desired experiments.
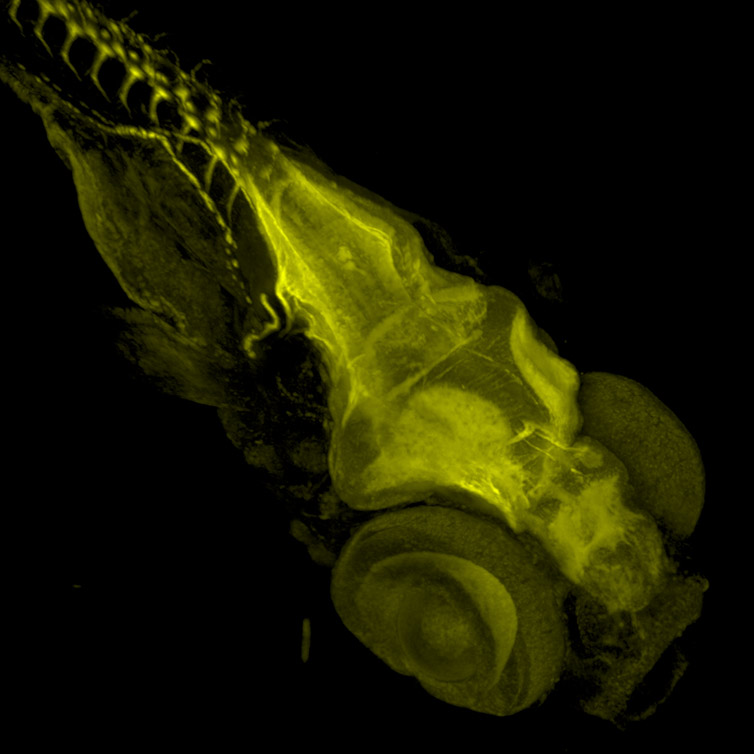
Figure 8 - Zebrafish stained with Acetylated Tubulin. Image acquired with Dragonfly spinning disk microscope using a Sona 4.2B6 sCMOS camera. Image courtesy of Marco Campinho, Universidade do Algarve.
| Options | Comments |
| LED | In confocal systems, selecting the imaging area of the sample is most often done visually through the eyepieces, as a result the user cannot use laser light sources, alternative options include metal halide lamps, Xenon bulbs or LED light sources. Currently LED light sources are quite good at exciting the samples at the desired wavelength, having the extra advantage that LED light sources deliver low energetic light, which will decrease photobleaching and phototoxicity when screening though the sample. LED light sources have long-time times and relatively low cost. For these reasons they have all but replaced Metal halide and Xenon light sources. The number of wavelengths required is also important to consider. It might be important to have multiple LED lines in a system, consequently, the more wavelengths available, the more expensive the light source will be. |
| Metal halide | |
| Xenon |
| Options | Comments |
| SIM | There are many different super-resolution techniques affording increased resolution, using variable laser intensities, optics and computer-based methods to achieve the improved resolution. Generally, the higher the laser power required for imaging, the less compatible the technique with live cell-super resolution. Here we discuss a range of super-resolution techniques and how easy they are to integrate within a microscopy system. The more complex the technique to implement then it is likely that the associated costs will be higher. SIM offers resolution down to 100 nm, and is compatible with widefield microscopy, and live-cell imaging. SIM works with any fluorophore and can achieve speeds up to 1 frame per second. However, SIM requires specialised optical components and delivers more overall power to the sample than traditional widefield methods. SIM requires a dedicated microscope and is not typically offered in a multimodal confocal system. STED delivers resolution in the range of 40 -50 nm. The optics of STED is complex and expensive and might not be accessible to all laboratories. In STED, the super-resolved image is delivered immediately during acquisition and does not require any computational image analysis. STED requires a dedicated microscope and is not typically offered in a multimodal confocal system. Single Molecule Localisation Microscopy (SMLM) techniques such as STORM/dSTORM and PALM rely on photoactivation of selected molecules to pinpoint their location. By repeating the excitation of a small number of molecules 103 to 104 times, a super-resolved image is delivered. STORM requires high computing power to analyse the images, but on the other hand, no special optics are needed, rendering it a lower budget option when compared to STED or SIM. SMLM technologies require higher laser powers and therefore are not compatible with live-cell imaging. STORM is a widefield technique and can also be combined with TIRF illumination. SMLM techniques (STORM and PALM) deliver the highest resolution of super-resolution technologies achieving up to 10 nm laterally. SRRF (Super Resolution Radial Fluctuations) and Andor’s SRRF-Stream+ is compatible with live-cell imaging. SRRF-stream+ is a low-cost camera-based method to achieve super-resolution and delivers 100 nm of lateral resolution. Higher resolution is possible depending on the imaging conditions. It does not require complex sample preparation and can deliver super-resolved images deep inside cells and tissues. The improvement in resolution of SRRF-Stream+ is comparable with that achieved using SIM, however SRRF-Stream+ delivers an easy more-affordable camera-based mechanism to achieve improved resolution for an existing system, where the camera can be swapped out, or built into a new system. |
| STED | |
| STORM | |
| SRRF-Stream+ |
Note: For more information please see the most popular microscopy techniques in the world today.
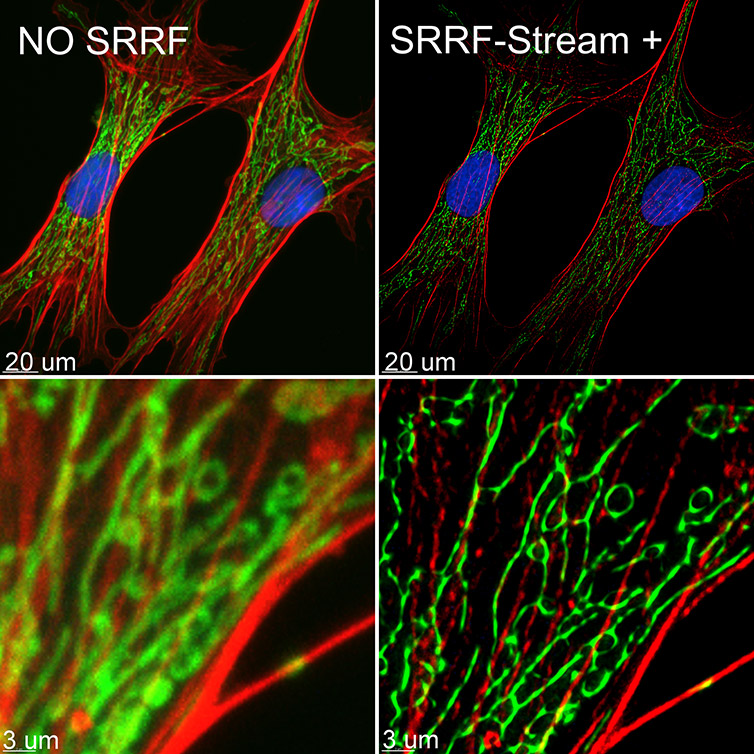
Figure 9 – BPA cells stained with phalloidin (red), mitotracker (green) and DAPI (blue) where imaged with Ixon 888 Ultra in confocal mode (no SRRF) and confocal mode with SRRF-stream+. Image acquired with Dragonfly spinning disk microscope using an Ixon 888 EMCCD camera. Image by Claudia Florindo, Andor, An Oxford Instruments Company, Belfast, UK
| Options | Comments |
| Astigmatic lens | If the user needs the maximum resolution delivered by dSTORM, then consider equipment that can deliver 3D super-resolved images. An elegant way to deliver 3D-dSTORM images is to use an astigmatic lens that causes a distortion in the PSF. The PSF distortion encodes a differential positive and negative axial offset in X and Y dimensions that can then be translated into an axial position, allowing a 3D super-resolved image to be created. Andor Dragonfly has an integrated astigmatic lens option that can be used to acquire 3D-dSTORM images. One should decide at the time of purchase if 3D-STORM is required. However, this option is upgradable post purchase, but the system will need to be returned to the factory increasing costs and resulting in a loss of experimental time and productivity. |
Extra Note: The PSF - Point Spread Function - is the response of an imaging system (e.g a microscope) to a point object. When light passes through an optical system (lens and other imaging components) is distorted due to the properties of the optical components of the system. The PSF is a measure of such distortion.
An infinitesimal point will not appear as a point the image will be blurred. The resulting image is said to be convoluted. Since the PSF is the same in all imaging space (e.g. in all the object to be imaged) a mathematical process to revert the blur can be used – this process is named deconvolution. Deconvolution will effectively remove the “fog from the image” revealing the true object with greater clarity.
| Options | Comments |
| Single colour | A TIRF microscope (total internal reflection microscope) allows the user to acquire very detailed images of an object close to the sample surface. TIRF microscopes are built with obstacles on the light path in such a way that will force the light to reach the edge of the slide in TIRF angle. One consequence of this type of illumination is that images can only be acquired at a very thin boundary between the glass/water interface. TIRF will allow imaging up to a maximum of 100-200 nm from the top of the sample. Furthermore, the high resolution provided by a TIRF system makes it useful for single-molecule imaging; therefore, TIRF imaging can be combined with dSTORM acquisition. TIRF is the ideal solution for analyzing live cell events at the cell membrane boundary such as: membrane dynamics, vesicle trafficking, endocytosis, exocytosis and any other events at the cell surface. If TIRF is a requirement, options to consider, are:
|
| Simultaneous dual colour |
Video 3 – Adhesion protein visualized by TIRF Microscopy using Andor Dragonfly. DLC-1 (deleted in liver cancer) is shown in green and talin in red. Image courtesy of Rebecca Kelly, Institute of Integrative Biology, and the CCI (Centre for Cell Imaging), UK.
| Options | Comments |
| Multimodal | A multimodal system that delivers high-quality images in all modalities is the most versatile choice for research laboratories as well as core facilities. The imaging requirements of a core facility are vast; a multimodal system will offer several imaging options where multiple different imaging projects run in parallel. In research laboratories, it is essential to remember that, as research results and discoveries progress, additional imaging approaches may be required. In such cases, a multimodal system will offer the extra versatility without the need to purchase extra equipment. Widefield systems can be exceptionally fast and gentle to the samples, and as a result are a good option for live imaging. Nevertheless, widefield systems do not offer optical sectioning, and for this reason, thick and highly scattering samples are not suitable. Widefield can be used with success in live-cell imaging of thin organisms or thin fixed cells. Therefore samples such as bacteria, yeast, microalgae and monolayer cells are good options to image in widefield. A confocal system is a microscope that can perform optical sectioning using the pinhole to discard out-of-focus light. There are 2 types of confocal microscopes:
Traditionally multipoint confocal could not image deep into samples due to pinhole crosstalk. Andor’s Dragonfly has optimal pinhole interspacing design, and therefore allows imaging up to a millimetres in depth in thick samples. Dragonfly has very efficient sample illumination being quite gentile for live samples and imaging in confocal mode can deliver speed as fast as 400 frames per second. TIRF imaging, is a specialised microscope technique directed to image live-cell-surface events or cell/substrate attachment interactions. For more information about TIRF read requirement 17. Please note that specialized optical components are required to allow TIRF imaging and are included when building the system. If a system does not have TIRF optical components built into it from the outset it will be extremely complex and costly to retroactively include TIRF functionality. Super-resolution, the ability to visualise structures smaller than 200 nm, can be essential in many biological projects. There are several options for super-resolution that have advantages and disadvantages for each, again, having more than one super-resolution option will offer versatility. For more information about Super resolution read requirements 15 and 16. Overall, a system that could combine widefield, confocal, TIRF and super-resolution capabilities delivering reliable multimodal imaging, would be a dream system. Andor Dragonfly is such a system, delivering exceptional quality and high speed in any imaging modality: widefield, confocal, TIRF and super-resolution options (dSTORM & SRRF-Stream+). When considering all the imaging modalities combined in one system, Dragonfly is an excellent cost-effective option. |
| Widefield | |
| Confocal | |
| TIRF | |
| Super resolution |
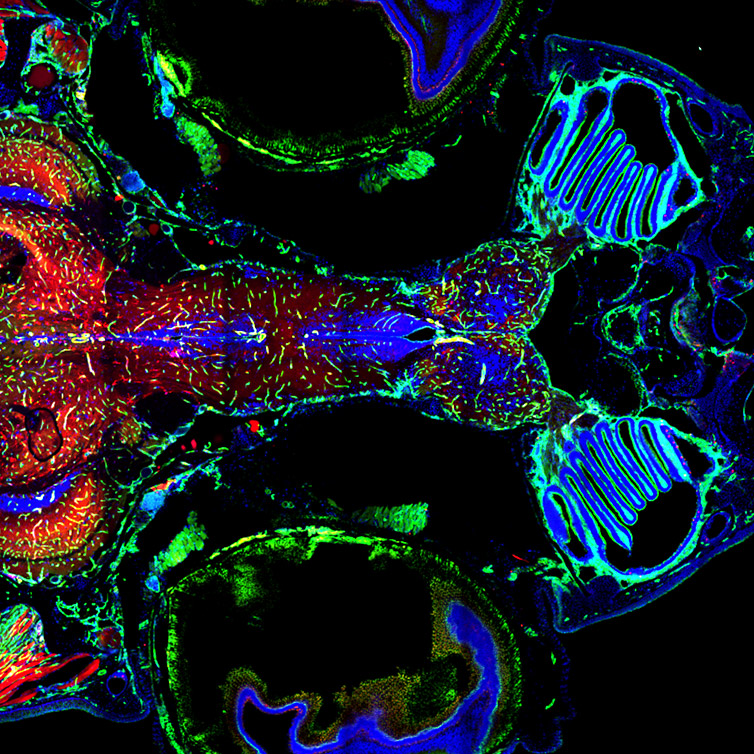
Figure 10 – Adult Zebrafish Brain and vasculature, Multi-tile image acquired with Andor Dragonfly 500 and using double camera simultaneous acquisition. Multiple tiles, stitching and in line deconvolution was performed during image acquisition. Image courtesy of Julien Rességuier at NorMIC (University of Oslo).
| Options | Comments |
| Image acquisition options | When acquiring a system, acquisition software must be considered for example, can the software integrate with the required imaging modalities and applications? What is the basic package of the software, and what are the extra modules? What extra modules are needed for your application requirements? And how much do the extra modules cost? Basic software packages of most confocal systems often include functionality for Z stack, time-lapse, multipoint, multichannel experiments and 6D experiments (3 channels, time, multimodal acquisition - different channels, time, and Z). Nevertheless, there are many more options that can be included in microscope acquisition software package, and these features can be standard or not. To name a few: tile /mosaic imaging, 2D and 3D stitching, deconvolution, real-time stitching, real-time deconvolution, multiwell integration, etc. The critical factor is to ensure that the software is powerful enough to allow the applications the user needs. For further flexibility, software that allows scripting and integration with external devices through scripting will ensure flexibility for new applications that might arise after the time of purchase. |
| Options | Comments |
| Image analysis options | As for software, not only the acquisition options but also the visualisation and analysis tools are significant. Ideally, the system should be integrated in such a way that the acquisition software and the analysis software can communicate with one another in order to create an efficient and productive workflow. The analysis package should ideally contain tools to deliver statistical data required to analyse the experimental questions. Examples of such are tools for analysing intracellular distances, intracellular spots (organelles), cell tracking (3D and 2D), neurite branching and spine density, cell cycle, etc. A deconvolution option is a must, and if not already included in the acquisition software, then it should be integrated into the analysis package. Why is deconvolution so important? The microscopic images are distorted by an optical process that is inherent to the way photons behave when passing through the glass. An infinitesimal point will not show itself as a point in an image, but it will have a dispersion characteristic of that optical system. There are ways to minimise significatively this blur, a very effective way to do so is to use a confocal. Nevertheless, there will always be some convolution, and therefore to have an image that truly represents the object, the raw data should be deconvolved. If working with a large organism or continuous samples in which multiple imaging tiles are acquired, then consider a stitcher as an extra module for the analysis package. The stitcher should quickly and effectively join all the tiles of our imaging together, allowing some overlap between the tiles to deliver a continuous image. The stitcher should be able to handle big data sets. The user should ensure that the stitcher can render the size of the data sets needed. If repetitive analysis is to be done, then batch analysis capabilities are important to consider, so that once the analysis parameters are defined, the analysis can be batch automated. In conclusion, the analysis and rendering software should deliver the statistical data but also, present the data in the best way possible, offering multiple rendering and visualisations modalities so that the data can easily and unequivocally show the results. Overall, as a rule of thumb the more modules, the more pricey will be the analysis module. Nevertheless, consider that when purchasing an excellent image analysis software, the extra price can be recovered in analysis time and in the superior output data quality. |
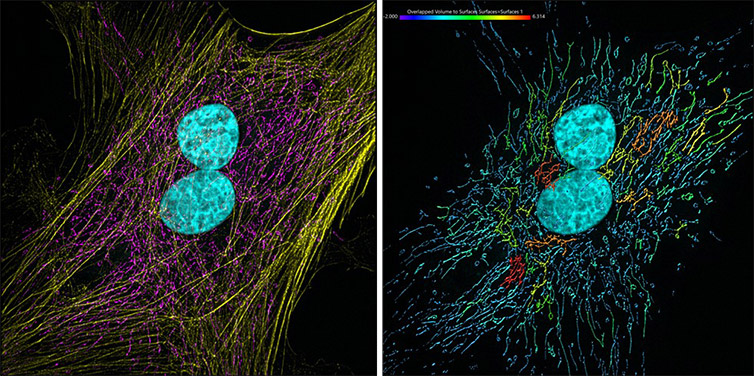
Figure 11 – Mammalian cells presenting actin, mitochondria and DNA. Image acquired with Andor Dragonfly 500, an iXon 88 camera and using SRRF-Stream+ imaging modality. Image was processed with Imaris 9.7. The actin filaments (yellow) were segmented with the Surface component. Image by Claudia Florindo, Andor, An Oxford Instruments Company, Belfast, UK
| Options | Comments |
| Basic coverage | Nowadays confocal microscopes are like an Orchestral Violin: a high-end piece of equipment that offers exceptional quality. Nevertheless, all high-end pieces of equipment need to be finetuned to continuously deliver the quality standards set at the time of purchase; for this reason, regular maintenance of cutting-edge instrumentation is a must. When purchasing the microscope, usually warranty is included in the purchase agreement. The buyer can opt to purchase an extended maintenance package immediately ensuring that the equipment would be finetuned at its maximum during the first years of use. After the initial warranty runs out, microscope companies offer maintenance packages that for simplicity can be divided into 3 types of packages: Basic, Medium and Full Coverage. The basic package usually includes a pre-defined number of site visits (to diagnose and solve problems) and a preventive maintenance visit, but no replacement parts are covered. The medium package includes the basic package plus the replacement of some equipment parts (in many cases excludes lasers replacements). The Full Coverage package is usually the most expensive one, but on the other hand is the one that ensures premium attendance, unlimited callouts to solve issues. In full maintenance coverage all replacements, travelling, consulting costs are covered, the company ensures a complete and priority attendance to the user. When budget allows a full coverage, this is a “headache-free” solution. Having a maintenance package that offers remote diagnose will save time and speed up the support process. As “time is money” consider choosing a support contract that offers remote diagnose, trusty and high-quality support. |
| Medium coverage | |
| Full coverage |
Figure 12 – Overall comparison of prices and coverage of maintenance packages.
