Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Viruses hijack many aspects of cell function in order to access the replicative machinery of the host-cell, and to create a suitable environment for their successful replication. One of the key steps is transport of the virus from the cell membrane within the cytoplasm, and to the nucleus. Microtubules are essential to intracellular transport processes within cells including that of organelles, secretory vesicles, other macromolecular components. It is also well researched that viruses also make use of the host-cell’s microtubule transport system.
The Polyomaviridae family are a group of small non-enveloped double stranded DNA viruses that are tumorigenic. A number of human pathogens of clinical significance have been identified to date such as BKPyV, JCPyV and Merkel carcinoma cell polyomavirus (MCPyV) which may affect immunocompromised individuals. Mouse polyomavirus (MPyV), a model for these pathogens, has a dsDNA genome that encodes six gene products, three of which are the early antigens (large, middle and small T) and three are structural proteins (major capsid protein, VP1, and minor capsid proteins, VP2 and VP3). The early antigens are involved in viral transcription and DNA replication and have been found to deregulate host cells to mediate virus replication. The protein capsid is icosahedral in structure with 72 capsomeres. Each capsomere is comprised of VP1 arranged into pentamers with either VP2 or VP3 minor proteins presented on the inside of the capsid. Capsomeres are formed in the cytoplasm, and then the complex is transported into the nucleus, where the assembly of virions takes place. Although interactions of the VP1 capsid protein is known to be involved in viral entry and trafficking of the genome to the cell nucleus steps, how VP1 interacts with cellular structures such as microtubules remains unclear.
The research group of Assistant Professor, Jitka Forstová, Head of the Laboratory of Virology in the Charles University have been using MPyV to help clarify the interaction of VP1 and microtubules. Their research has shown that microtubules (including the mitotic spindle) and the cellular chaperone heat shock protein 90 (Hsp90) and other components interact with VP1. During the late phase of infection, it was possible to observe VP1 in the cytoplasm in association with microtubules.
In their recent publication in Viruses (2020), “The Major Capsid Protein, VP1, of the Mouse Polyomavirus Stimulates the Activity of Tubulin Acetyltransferase 1 by Microtubule Stabilization”, Forstová and colleagues have been able to explore these interactions in much greater depth. This included looking at the binding of VP1 to microtubules, and the impact of stabilization of the microtubules and acetylation of tubulin αK40. In addition, they investigated αK40 acetylation and the role this plays in infection by MPyV. They have exploited a combination of techniques - including as Immunoelectron Microscopy, confocal and TIRF for imaging of the fluorescently labelled proteins and microtubules, as well as inhibition assays and in situ cell fractionation to determine these complex interactions effectively.
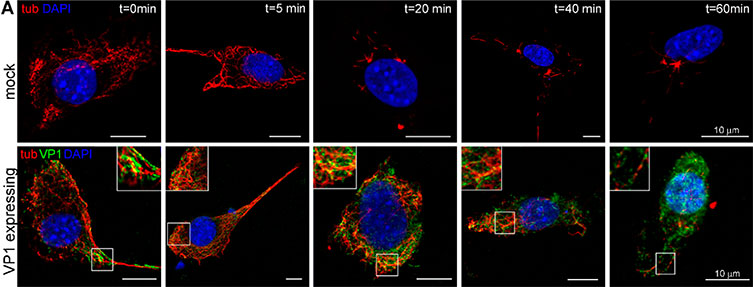
Figure reproduced Lenka Horníková et al. VP1 protects microtubules from nocodazole induced depolymerization. WOP cells were transfected with plasmids expressing VP1 and 24 hpt cells were treated with nocodazole (4 µM) for the indicated times. (A) After the treatment, soluble tubulin was washed out, the cells were fixed, and polymerized tubulin (tub; red) and VP1 (green) were stained by specific antibodies. Shown are the selected confocal sections. Enlarged details of the cells are presented in insets. Bar: 10 µm.
This research has helped to reveal some of the intricate interactions involved between VP1 and microtubules during MPyV infection. To summarize some of their findings: they found that the number of microtubules was found to be increased in cells that express VP1, and that VP1 binds directly to the microtubules and provides a stabilizing effect. VP1 was also found to stimulate the hyperacetylation of microtubules with microtubule acetylation being found to increase during the late phase of infection. Interestingly, acetylated microtubules are not necessary for viral infection.
Histone deacetylase 6 (HDAC6) is a protein with a wide-ranging functionality. It has a deacetylase activity for α-tubulin as well as chaperone proteins, it also possesses a ubiquitin binding domain at its C-terminus that indicates a protein metabolism function. Although in this study they did not observe that HDAC6 was involved in microtubule acetylation, the results do show that HDAC6 is involved in the late phase of MPyV infection. Inhibiting HDAC6 using tubacin resulted in increased levels of VP1 and a corresponding decrease in the production of viral progeny. The role of HDAC6 may not necessarily be directly connected with microtubule acetylation, but given the wide range of function of HDAC6 it may act via another inhibitory or activation process. Further studies will undoubtably help our understanding the complex interactions of VP1 and microtubules and potentially lead to possible anti-viral targets and strategies for the Polyomaviridae group of viruses.
For their confocal and TIRF Imaging they used a Nikon, Ti-E Eclipse microscope with either 100× or 60× 1.49 N.A. oil immersion objective (Nikon, Plan Apo) with an iXon Ultra 888 EMCCD camera switching between 561 nm and 488 nm laser and the corresponding filter set for imaging of the Rhodamine-labeled microtubules and EGFP-labeled proteins. The acquisition rates required were one frame per 10 seconds and used flow cells. This configuration using an iXon Ultra 888 EMCCD camera is highly suitable for these studies since the signals from fluorescently labelled proteins are often very weak and need the most sensitive detector possible. Find out more about how the iXon camera for virology studies in our solution note.
Research paper: https://www.mdpi.com/1999-4915/12/2/227/htm
The Laboratory of Virology is part of the Department of Genetics and Microbiology, Faculty of Science at the Charles University. One of the main areas of study of the Laboratory is the biology of small non-enveloped DNA viruses such as mouse polyomavirus the type species of the Polyomaviridae family, as has been described in this article. A part of their work is in the production of polyomavirus-based nanoparticles (virus-like particles, VLPs) for basic virology research as well as for therapeutic and diagnostic purposes. VLPs are particularly interesting as they can be used in many ways such as potentially vaccines, but also to transfer exogenous genetic information effectively to specific target cells. The Laboratory is also actively engaged in vaccine research and development against porcine circovirus and bovine papillomaviruses.
Find out more about the research of the Laboratory of Virology, Department of Genetics and Microbiology, Faculty of Science, Charles University, Czech Republic: https://www.natur.cuni.cz/biology/genetics/veda-a-vyzkum-1/laboratory-of-virology/laborator-virologie-en
Date: Aug-20
Author: Dr Alan Mullan
Category: Case Study
