Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Caged compounds act as light-sensitive probes that functionally encapsulate biomolecules rendering them inactive. By applying targeted illumination, it is possible to “uncage”, that is to release and activate the biomolecules within at a precise time point, and then study the effects that follow. This can be a very powerful means to study the localized effects of dynamic biological processes such as signalling pathways. Illumination within the blue to UV spectrum is typically used to uncage the biomolecules and initiate the experiment, coupled with fluorescence microscopy to permit the observations that follow. The illumination intensity is typically at a higher power density than is used for photoactivation, photoswitching or photoconversion experiments.
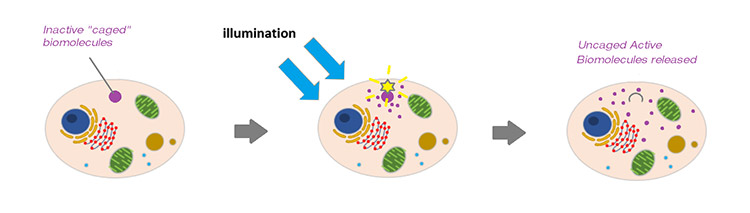
The precise, timed control of illumination power and wavelength of illumination to region(s) of interest is a vital part of uncaging biomolecules. Mosaic and MicroPoint are two products that are suitable for photostimulation experiments, each having some benefits that suit particular usage scenarios.
Date: February 2023
Author: Dr Alan Mullan
Category: Solution Note
