Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
The resolution in light microscopy is limited by the diffraction limit of light, which means that structures that are closer than 200 nm apart cannot be distinguished unless researchers use Super-resolution techniques. An alternative approach to super-resolution methods was developed by Edward Boyden's laboratory at MIT in 2015 (Chen et al., 2015). Instead of increasing the optical resolution of the microscope, Expansion Microscopy "expands" the sample isotropically. This technique has been gaining a lot of attention not only because it is an alternative approach to super-resolution, but also because it can be combined with current super-resolution techniques and increase even further the limits of what the microscopes "can see". For a full summary of the protocols of expansion microscopy, please read our technical article - What is Expansion Microscopy?.
Nevertheless, there are challenges to overcome to successfully image expanded samples. One of the main challenges is the loss of fluorescence induced by the different steps of the protocol. The complexity of the fixation procedure applied to the organelles causes problems in antibody labelling, leading to samples with a low signal. During the expansion procedure monomers of a polymer are delivered into the cells, and then, once inside, the polymerisation is triggered (gelation). During gelation, the inefficient crosslinking results in fragmented signal preservation in the hydrogel. Next, the homogenisation step aims to avoid sample distortion and ensure that the specimen organisation is kept intact. However, the fluorescence signal can decrease up to 60% during homogenisation. During the actual expansion, a 4-fold expansion means a 64-fold volumetric expansion; hence the fluorophore density and brightness of the fluorescence signal is lowered by 64x. Therefore, imaging expanded samples requires an instrument that is sensitive enough to detect low light signals. Other challenges consist of dealing with the expansion itself including imaging a very large field of view (Figure 1.), and imaging deep into the sample. These requirements are problematic and difficult to achieve with conventional microscopes.
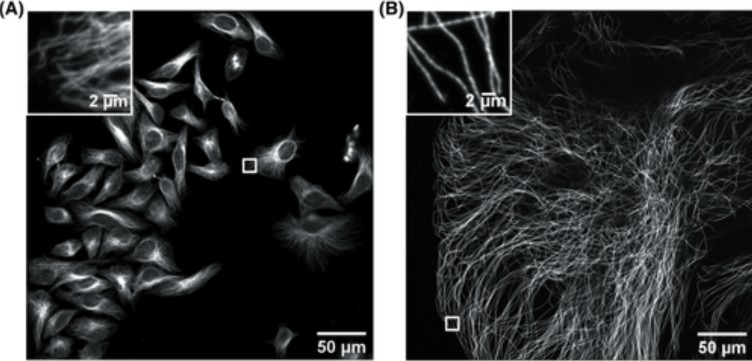
Figure 1. Confocal image of HeLa cells non expanded microtubules (right) and 4.5x linearly expanded microtubules (left). Imaging was performed on an Andor high speed spinning-disk confocal microscope (Dragonfly) with a 40×, numerical aperture (NA) 1.15 water- immersion objective. (A) Confocal image of HeLa cells with immunostained microtubules, imaged at a single xy plane at the bottom of the cells. The inset in the upper left zooms in on the small box at the middle right. (B) Confocal image of a ∼4.5× linearly expanded HeLa cell with immunostained microtubules, imaged at a single xy plane at the bottom of the cell. The inset in the upper left zooms in on the small box at the bottom left. Scale bars in (B) indicate post-expansion scales. Only a fraction of an expanded cell fills the entire field of view. The respective insets display a zoom of the respective small boxes of the full field of view. (Zhang, C., et al, Current Protocols in Neuroscience ,2020).
To overcome the loss of signal in expanded samples, a highly sensitive acquisition system is needed. Researchers need to use a microscope that can facilitate optimal delivery of light to the samples and extremely efficient capture of all the photons from the sample, i.e. high QE detectors. The use of highly sensitive cameras turns camera-based confocal systems into the ideal solution to capture all signals from expanded samples, even the dimmest ones.
In addition, to image expanded samples with high productivity, a large field of view of acquisition is a must-have. A multipoint confocal microscope that delivers images in an instant with a large FOV of acquisition is, therefore, the perfect solution. The multipoint confocal will need to have optimised pinhole spacing to avoid pinhole-crosstalk, allowing imaging deep into thick expanded samples. Moreover, thick expanded samples are big, and on many occasions, multiple tiles are required to be able to gather all the three-dimensional information on the sample like the example displayed in figure 2 and video 1. Ideally, the system should have a very flat uniform illumination that when used in combination with a software, can deliver seamless stitching.
Video 1. Mouse Hippocampus Expanded sample captured with Andor Dragonfly rendered in Imaris. In the video the researchers took advantage of the large dragonfly FOV and of Borealis highly Uniform illumination. The result delivered a perfectly stitched Mouse hippocampus movie where the detail on the brain cell can be clearly visualized. Courtesy of Jennifer Santini, UCSD School of Medicine, Microscopy Core Facility
The use of multiple wavelengths is an important requirement that will allow users to label the numerous targets in the sample and obtain information on localisation of different structures and how they relate to each other. The use of Near Infra-Red (NIR) wavelengths is an extra bonus, and not only extends the fluorochrome pallet but, very importantly, NIR wavelengths penetrate deeper into the sample.
Additionally, imaging the expanded sample using super-resolution techniques will afford improved resolution. For example, combining Expansion Microscopy with SRRF (super-resolution radial fluctuations) will give an increase of at least an extra 2X on the resolution obtained with the expanded sample alone.
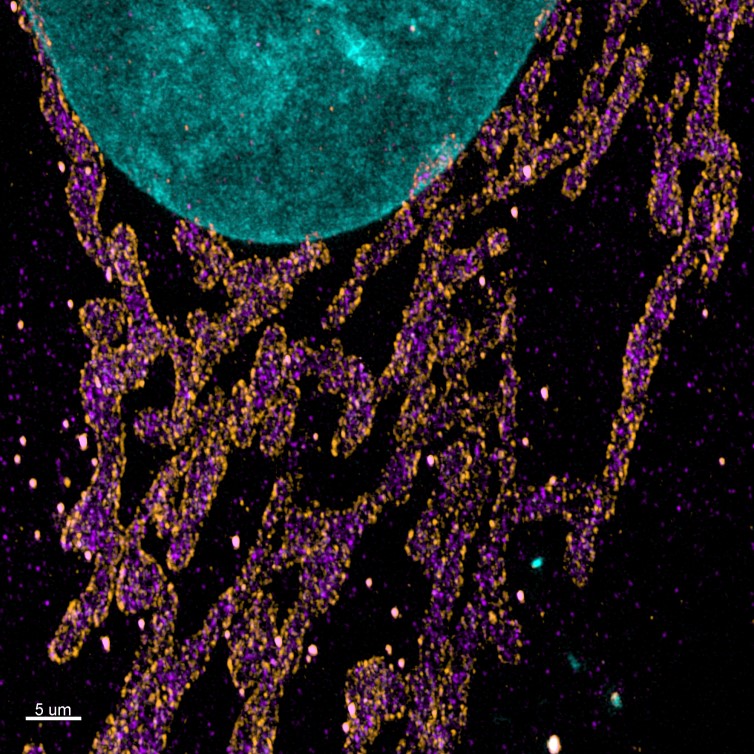
Figure 2. Maximum projection images of the mitochondrial import receptor. Cell where labelled using the post-expansion protocol with HSP70 (cytosolic side of organelles membrane - purple), TOM20 (outer mitochondrial membrane - orange), DAPI (nucleus - Cyan), and image with Andor Dragonfly. Courtesy of Line Verckist,University of Antwerp.
Andor recommends the Dragonfly confocal equipped with either the iXon Ultra 888 back-illuminated EMCCD, or the Zyla 4.2P sCMOS cameras for expansion microscopy. The dual lens spinning disk system, combined with highly sensitive EMCCDs and sCMOS detectors delivers a high-quality image even in samples with weak staining. Dragonfly has optimised pinhole spacing which allows imaging deep into samples without pinhole crosstalk. Further, Andor's patented Borealis illumination delivers uniform illumination across the field of view, which is a key factor for imaging the multiple tiles on a large expanded sample. Combined with a large field of view,22 mm diagonal, Dragonfly can deliver fantastic images with remarkable productivity.
In the following table, we present how dragonfly spinning disk confocal addresses the current and future challenges in imaging expanded samples
| Key Requirement | Solution for Expansion Microscopy |
| Image large samples | Expanded specimens are larger than the objective field of view, a microscope that can acquire fast and deep is required. Dragonfly is an instant confocal, 10 times faster than point scanning confocal. Dragonfly has a very wide field of view with 22 mm diagonal. The internal beam splitter of the Dragonfly allows simultaneous acquisition of 2 channels with 2 independent cameras. Result 1 - Acquire images at high speed up to 400 frames per second – increase productivity. Result 2 - The large FOV allows to acquire more data on a single image – increase productivity. Detect two independent channels simultaneously without compromising speed or resolution. |
| Image thick samples | Post expansion the specimen thickness is increased. The expansion microscopy specimens consist largely of water, the refractive index heterogeneities of cells and tissues typically causing optical aberrations are now negligible and deep imaging is straight forward [13]. Dragonfly excels in imaging thick specimens such as organoids, brain tissue or embryos. The custom pitch of the spinning disk pinholes leads to a reduced crosstalk between pinholes and the 25 µm pinhole is ideal for imaging thick samples as this option provides the best axial resolution. Result 1 – Imaging with detail in all three spatial dimensions. Result 2 – Image through hundreds of microns axially with great axial resolution & high contrast. |
| Homogenous illumination & extended spectral Range | Expanded samples often require the acquisition of multicolour 3D montages, the uniformity of the illumination field is essential to obtain perfect montages. Andor Borealis™ patented illumination technology offers uniformity across the FOV. The ILE laser engine supports a broad range of excitation wavelengths from the UV to the NIR (400-800 nm). A GPU accelerated stitcher integrated within the Fusion software can be used to acquire and produce super-fast and seamless montage reconstructions. Result 1 – Increase in image signal by at least a factor two. Result 2 – Acquire multiple tiles and build a montage. Result 3 – Immediate visualisation of your data: build your stitched image while acquiring. Result 4 – Extended spectral range: greater flexibility in fluorochrome selection. Result 5 – Use NIR wavelength for deeper sample penetration. |
| Detect faint signals & deliver high-quality data | One of the main challenges posed by ExM is its impact on fluorescence signal. EMCCD and sCMOS cameras used with the Dragonfly offer the high sensitivity and quantum efficiency with 16-bit dynamic range. Result 1 ‐ High sensitivity – detect very faint signals. Result 2 ‐ 16 bit corresponds to 65536 grey levels. The user can acquire all the signals in a single image, from the very faint to the brightest. Result 3 ‐ High quantum efficiency – capture nearly all the photons from your sample. |
| Increase Lateral Resolution | Expansion microscopy is good alternative to super-resolution. However, this technique has also proven to unveil even more details that were previously unseen when combined to super-resolution [8, 14]. Since samples are expanded and thick, a super-resolution technique that can acquire deep inside cells or tissues is desirable. Andor's EMCCD and back-illuminated sCMOS cameras offer an integrated license for SRRF (Super Resolution Radial Fluctuations Andor's built-in SRRF-stream algorithm allows real-time SRRF calculations and immediate visualisation of the super-resolved images. SRRF is compatible with conventional fluorophores, and there is no need for complex sample preparations or special optics. SRRF is compatible with confocal, TIRF and widefield imaging, and with deep imaging. Result 1 – Additional increase in lateral resolution in resolution of your expanded samples (between 2- and 6- fold). Result 2 – Obtain extra resolution without any further sample preparation. Result 3 – The extra increase in sample resolution can be acquired tens of µm deep inside the tissues. |
| Multiplexed imaging | The aqueous nature of expanded specimens and the spacing out of biomolecules may open the door for highly multiplexed imaging. Dragonfly is an excellent solution for multiplexing in situ hybridisation thanks to the recently incorporated REST-API. Fusion's REST-API allows Dragonfly to be easily triggered for imaging and synchronised with other devices for multiplexing. Result 1 – Automatic control of the multiplexing set-up. Result 2 – Flexible protocols that can be adjusted during the experiment. |
References:
Date: Oct-20
Author: Dr Claudia Florindo & Dr Sébastien Bellow
Category: Solution Note
