Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
TIRFM makes use of an optical effect that can be adapted to observe fluorescent events occurring at the interface between two optical media of different refractive indices. Excitation light incident upon such a boundary, travelling at an angle greater than the critical angle, undergoes total reflection. The electromagnetic field of the total internal reflected light extends into the sample beyond the interface, extending only a few hundred nanometres into the second medium of lower refractive index - essentially in the z direction. Furthermore, this evanescent field decreases exponentially in intensity along the z-axis of penetration. Only the section of the specimen located within the evanescent field undergoes fluorescence excitation. TIRFM is limited to the area within a few hundred nanometres of the glass/sample interface, where total internal reflection is occurring.
TIRFM is an increasingly popular technique for visualizing, with high signal-to-background ratio, processes that occur in and around the membrane of living cells (partially due to the availability of novel membrane-specific fluorophores). In contrast to the spinning disk confocal technique which can yield rapid optical sections of approx. 1 μm thickness, the excitation volume of a TIRF evanescence field extends only approx. 100 nm into the sample, the intensity of which decays exponentially across this distance. This gradient can later be translated into movements (in z axis) of fluorescently labelled organelles being trafficked from and to the plasma membrane. Since only a very thin sliver of excitation is being produced, we only detect photons that are created within that excitation volume, which has the effect of significantly improving signal-to-background. There are however, other sources of background photons in TIRFM that must be taken into account, and minimized where possible, as described below.
Whilst a thin excitation volume of the order of 100 nm depth sounds very useful, it must be remembered that due to the nature of the TIRF effect, we cannot step this excitation plane through the cell. TIRFM is a surface interface phenomenon, and as such is restricted to studying sample that is within 100 nm or so of the glass interface of the slide. In living cells therefore, TIRFM is ideal for capturing high resolution, high Signal-to-Noise (S/N) kinetic series of membrane events, such as exocytosis and membrane receptor/transporter lateral dynamics. TIRFM is also a common technique for imaging single molecules dynamics, covered more thoroughly in the Single Molecule Detection area of the Biology Applications section.
Two common approaches to TIRFM have evolved, often known as objective-type and prism-type, each illustrated below. The objective approach carries the key advantage that the sample is easily placed and is accessible for manipulation, e.g with patch-clamp pipette. The approach requires that the laser be introduced through the microscope, and the angle over which TIR can be practically achieved requires use of an oil-immersion objective with a Numerical Aperture (NA) >1.4. However, the objective configuration can result in signal-to-background limitations, involving a combination of autofluorescence from immersion oil and stray reflections from the excitation laser which is total internally reflected back through the objective again.
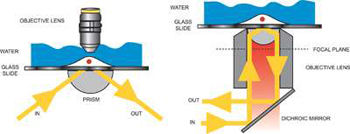
Illustration of prism-type TIRFM (left) and objective-type TIRFM (right)
The prism technique is easy to set-up, although it carries the disadvantage that the sample is located between the objective and the prism. As a consequence, more elaborate methods of sample placement and manipulation often have to be considered. The excitation area with prism technique is potentially wider, typically up to 100 μm beam waist, the illumination field is flatter and while the signal is lower with the prism technique, so is the background photon noise, considerably so, since with the prism there are no stray reflections of the excitation light as with the objective TIRFM. One can also potentially avoid use of immersion oil objectives and their associated autofluorescence. The net effect is that signal-to-background ratio is potentially better with the prism approach, a very important point to bear in mind when using Andor's deep-cooled iXon3 EMCCD camera technology, which offers negligible instrumental noise. Given the relative advantages and disadvantages of each configuration, many TIRF experimentalists opt to set up both configurations in their laboratory, using whichever one is more appropriate to a given application.
It is often of interest to dynamically image multiple different biomolecules, and their interactions within the sample volume. The TIRFM technique can readily be extended to image multiple fluorophores labels, through integration of a multi-line laser system, preferably a solid-state laser solution with Acousto-Optical Tunable Filter (AOTF) modulation. This technique can be readily adapted for FRET analysis, preferably through integration of a suitable beam splitting device on the emission side.
A live cell microscopy set-up that enables one to switch rapidly between TIRFM and widefield epi-fluorescence microscopy, the latter for deeper penetration of the excitation into the bulk of the sample, represents a flexible and powerful approach for studying intracellular transport mechanisms. In Dr Derek Toomre′s CINEMA laboratory at Yale School of Medicine, such a combined approach is being used to visualize vesicles as they progress through the cytoplasm and eventually fuse with the cell membrane. Specially designed optoelectronic systems that take advantage of acousto-optic modulators have been assembled in several labs, acting in synchrony to oscillate illumination between TIRF and epi-fluorescent widefield.
