Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Science has limits imposed by the laws of physics that constrain discoveries and the advancement of knowledge. In microscopy, up until the beginning of the twenty-first century, the diffraction limit of light was an unbreakable barrier. This law of physics dictated that two points could not be resolved (clearly separated) if they are closer together than half the wavelength of light used to view them. In practice, this would mean that light microscopes could only resolve objects/structures that are separated by 200 nm or more. The 200 nm barrier would therefore leave a significant gap of knowledge to be uncovered, since many subcellular structures and organelles are smaller than 200 nm.
In the first decade of the twenty-first century, the diffraction limit of light was overcome using a number of ingenious microscopy techniques. A new field of discovery was ready to be revealed. Several methods for super-resolution evolved, allowing imaging beyond the diffraction limit of light. Super-resolution microscopy methods such as STED (stimulated emission depletion microscopy), STORM/PALM (Stochastic optical reconstruction microscopy)/ (Fluorescence photo-activation localisation microscopy) and SIM (Structured illumination microscopy) have become available for researchers.
Although the Super-resolution methods have opened a new field of discovery, some limitations on these methods existed. Complex sample preparation, long acquisition times as well as high energy requirements for acquisition, render these techniques inappropriate (if not incompatible) for use with live-cell imaging. Furthermore, the optical requirements and computer power needed render these SR methods extremely expensive and inaccessible to many labs.
More recent advances with spinning disk confocal technology combining optical over-sampling with computational reassignment do achieve a 1.6x improvement beyond the diffraction limit and are suitable for live-cell imaging, but at the significant cost of a reduced field of view, and starting from a lower native resolution. Additionally, it is still inextricably linked to the high-cost hardware.
The Dragonfly spinning disk confocal with its unique combination of pinhole size and custom pinhole pitch separation has an improved native axial and lateral resolution, delivering better resolution for routine imaging. Then, using photon reassignment (deconvolution) on the acquired images, the final obtained axial and lateral resolution of the dragonfly can be 240 nm and 139 nm respectively.
But what if a researcher needs more resolution? Or does not have a spinning disk confocal microscope? Is there an alternative method that is fast, compatible with live imaging, and that can still acquire super-resolved images at the full field of view and, if required, deep inside the cells? The answer to these questions is yes, with SRRF or SRRF-Stream+ (Figure 1).
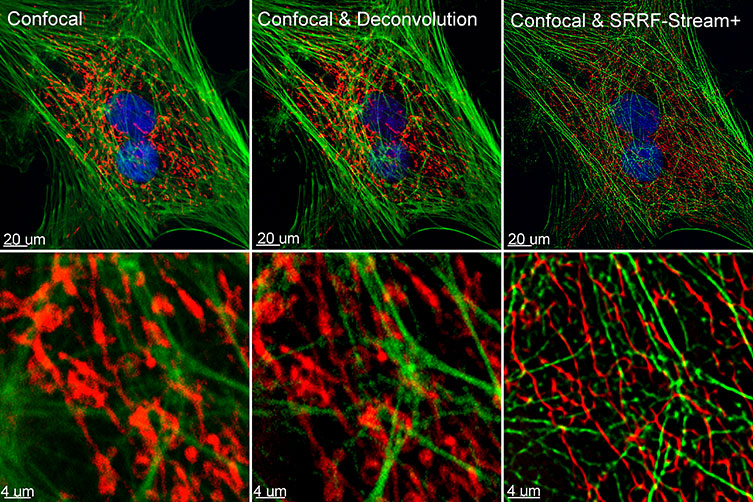
Figure 1 – Comparison between confocal imaging, confocal imaging & deconvolution and confocal imaging & SRRF-stream+. High magnification close ups are present for a better visualisation of the obtained resolution. BPA cells stained with phalloidin, mitotracker and DAP image with Ixon 888 in confocal mode (no SRRF) and confocal mode with SRRF-stream+.
In 2016, the Henriques lab developed an alternative approach to super resolution named SRRF – Super-Resolution Radial Fluctuations (1). SRRF can be combined with widefield, TIRF or confocal, and the final resolution will depend on the proprieties of the acquired data sets. Using the SRRF algorithm, the researchers can achieve resolutions in XY up to 50 nm (1). Furthermore, the SRRF algorithm does not require special sample preparation or special fluorophores for acquisition, being compatible with conventional fluorophores and fluorescent proteins.
Most importantly, a super-resolved SRRF image can be acquired by capturing on average between 20-100 frames (more frames will result in improved resolution) and the energy required for this imaging is in the order of the mW to W per cm2 range, making SRRF compatible with live-cell imaging. (1, 2) SRRF was initially provided as an ImageJ plugin. In order to obtain a SRRF image, a long acquisition workflow needed to take place and the SRRF-image was obtained by post-acquisition processing of the acquired data in the image J plugin – NanoJ.
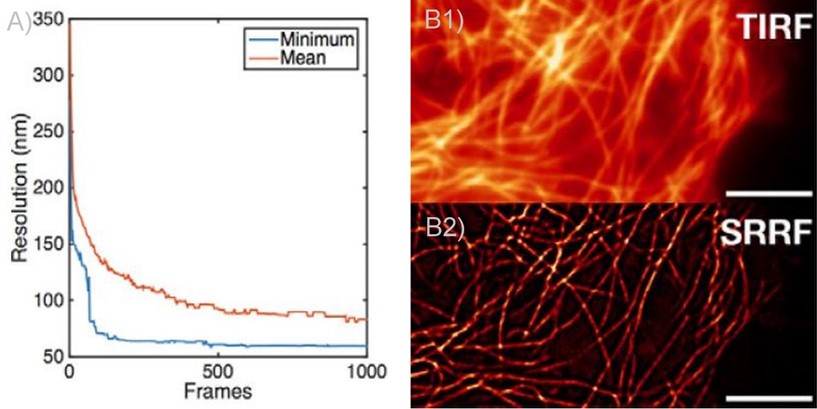 Figure 2 - SRRF and SRRF-stream resolution increase with the number of frames acquired (and processed) per time point. A) Graph presenting the resolution increase with the increasing number of acquired frames. As it can be observed there is a high increase up until 100 frames. Mean presents the average of all radial fluctuation correlation analysed (in different Regions of interest - ROI). Minimum shows the maximum resolution achieved for a given ROI. B1 and B2) Actin filaments imaged on Andor Dragonfly using TIRF (B1)) and TIRF with on the fly SRRF-stream processing (B2). It can be observed even when acquiring in TIRF modality, that SRRF-Stream provides a significant increase in resolution.
Figure 2 - SRRF and SRRF-stream resolution increase with the number of frames acquired (and processed) per time point. A) Graph presenting the resolution increase with the increasing number of acquired frames. As it can be observed there is a high increase up until 100 frames. Mean presents the average of all radial fluctuation correlation analysed (in different Regions of interest - ROI). Minimum shows the maximum resolution achieved for a given ROI. B1 and B2) Actin filaments imaged on Andor Dragonfly using TIRF (B1)) and TIRF with on the fly SRRF-stream processing (B2). It can be observed even when acquiring in TIRF modality, that SRRF-Stream provides a significant increase in resolution.
In 2018 after collaboration between Andor and professor Henriques, Andor launched SRRF-Stream. SRRF-Stream is Andor´s implementation of the SRRF algorithm that delivers super-resolution images on the fly with the click of a button. As with the original SRRF algorithm, the resolution of SRRF-Stream will improve with the number of frames captured per given point in time. This is most striking up to 100 frames, reaching a more stable increase from 100 frames to 500 frames, and a very modest improvement in resolution from 500 frames onwards (figures 2, 3). Please note that other factors will also affect resolution such as exposure time, Nyquist sampling, radiality magnification and the ring radius increase; these should be tested in order to optimise results.
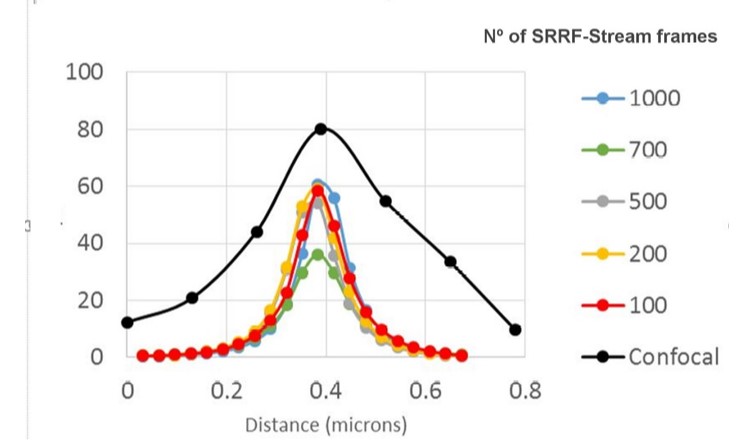
Figure 3 - SRRF-stream resolution increase with the number of frames acquired per time point. 100 nm tetraspeck beads where imaged and the FWHM was measured. We can observe that with 100 SRRF frames acquired the FMHW of the tertaspeck beads is 100 nm. The 100 nm bead can be accurately measured using the SRRF-stream algorithm and acquiring 100 frames. Images were acquired in confocal mode, and in confocal mode with different SRRF-stream acquired image images per point, respectively: 100, 200, 500, 700, 1000. Thanks to Dr Ann Wheeler, Head of the Advanced Imaging Resource at the MRC Institute of Genetics and Molecular Medicine, University of Edinburgh.
ANDOR´s SRRF-Stream was a major improvement allowing live-cell super-resolution, super-resolution deep inside cells or tissues way beyond the top of the coverslip.
SRRF-Stream is available with iXon Life and Ultra EMCCD and sCMOS cameras from the Sona series as well as the sCMOS ZL41. SRRF-stream is available through micro-manager and Fusion which is the control software for Dragonfly confocal. The advantages of SRRF-Stream are (3,4):
SRRF-stream is, therefore, a cost-effective solution for any microscope since all of them can be converted into super-resolution microscopes. SRRF-stream is compatible with any imaging modality, namely widefield, TIRF and confocal (Figure 4).
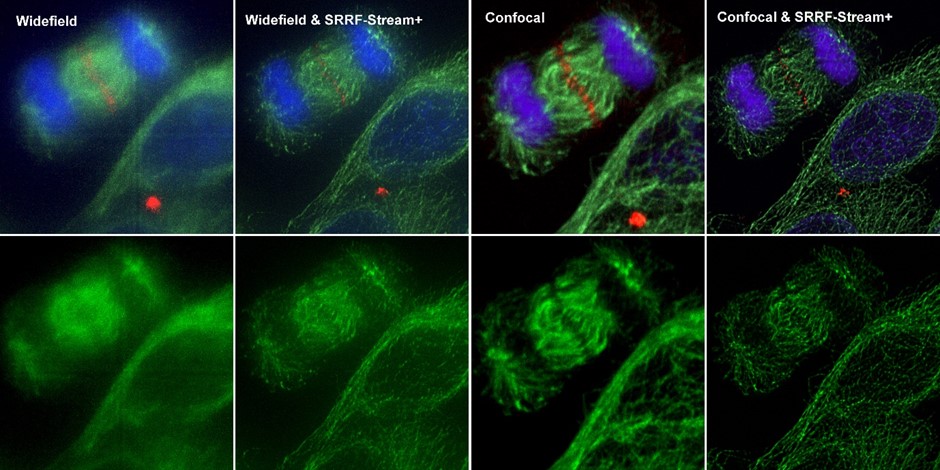
Figure 4 – Comparison between different imaging modes (widefield and Confocal) with and without SRRF-Stream+ Hela cells stained for MLKP1(red), a-tubulin(green-microtubules) a and DAPI (blue-DNA) were image in the Dragonfly with an Ixon888 camera.
In July 2020 we announced an additional improvement to our SRRF-Stream algorithm: SRRF-Stream+ (figures 1, 4, 5, 6, 7).
SRRF-Stream+ goes a step further with increased radiality measurements. On the previous version of SRRF, the radiality measurements were computed in 6 directions and now are computed in 24 directions (Figure 5). This enhancement will improve the result of your super-resolved processing data, and the occasional star artefacts previously visible, especially in circular structures such as kinetochores, are now removed (Figure 7).
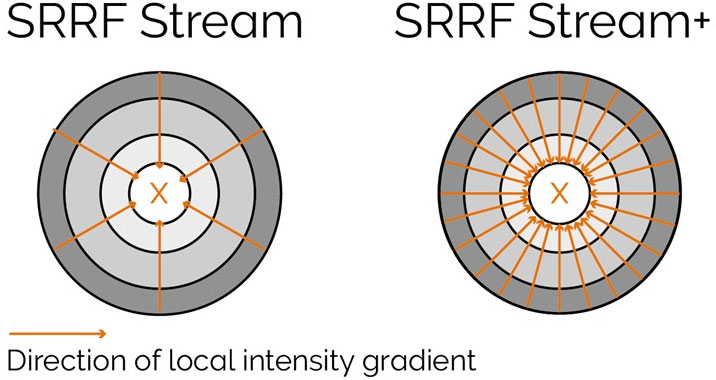
Figure 5 – Radiality computation measurements in SRRF-Stream and SRRF-Stream+ As it can be observed by the image the increased computational radiality measurements will deliver a more accurate result of the SRRF image.
To get the best from SRRF-Stream+, as before, the user will need to acquire images under Nyquist criterium. As previously reported, the major benefit in resolution will be obtained when oversampling by a factor of 2.3 or higher. Still, when oversampling by a factor of 1.5 good results can yet be obtained using the SRRF-Stream+.[1]
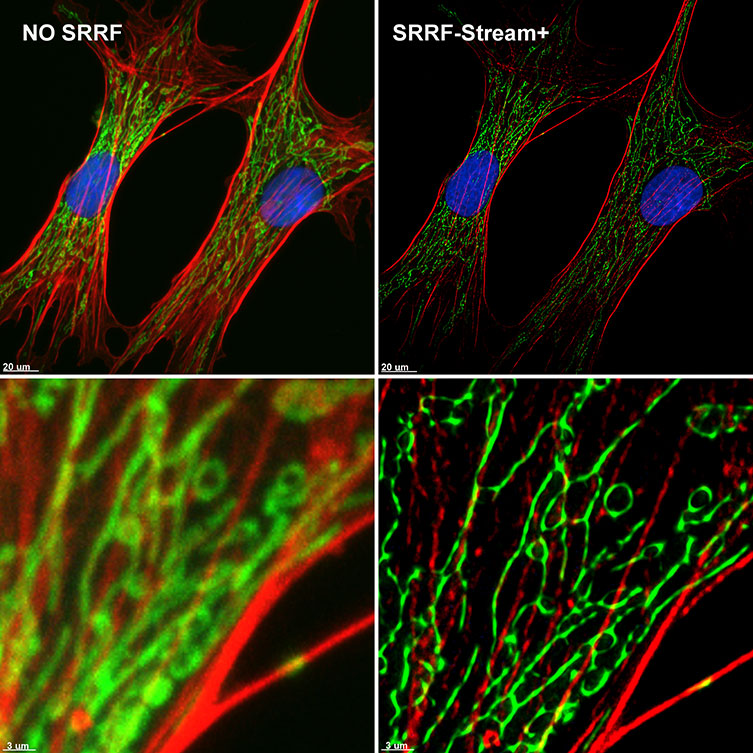
Figure 6 – SRRF-Stream+ delivers High-quality Super-resolved images. BPA cells stained with phalloidin (red), mitotracker (green) and DAPI (blue) where imaged with Ixon 888 Ultra in confocal mode (no SRRF) and confocal mode with SRRF-stream+
When analysing Figures 1, 4, 5 and 7 the benefit on SRRF-Stream+ is obvious, but one question will come into the mind of many potential users: How is the speed of acquisition affected? If SRRF-stream+ computes radialities in 24 direction instead of 6; is SRRF-Stream+ 4X slower than the original version of SRRF? When developing the new SRRF algorithm, Andor took into account that speed of acquisition is also an essential parameter to many researchers. It has been possible to achieve the improved image quality with a minimal impact to the acquisition speeds. To achieve this, CUDA performance has been optimised, perfectly utilising the massive computing power of nVidia graphic card to speed up SRRF-Stream+ calculations. As a result, a time-lapse of 100 time points with 50 SRRF-stream+ images per time point (corresponding to 500 images in total) will only vary by 0.5 secs in acquisition when comparing with our original SRRF-stream[2].
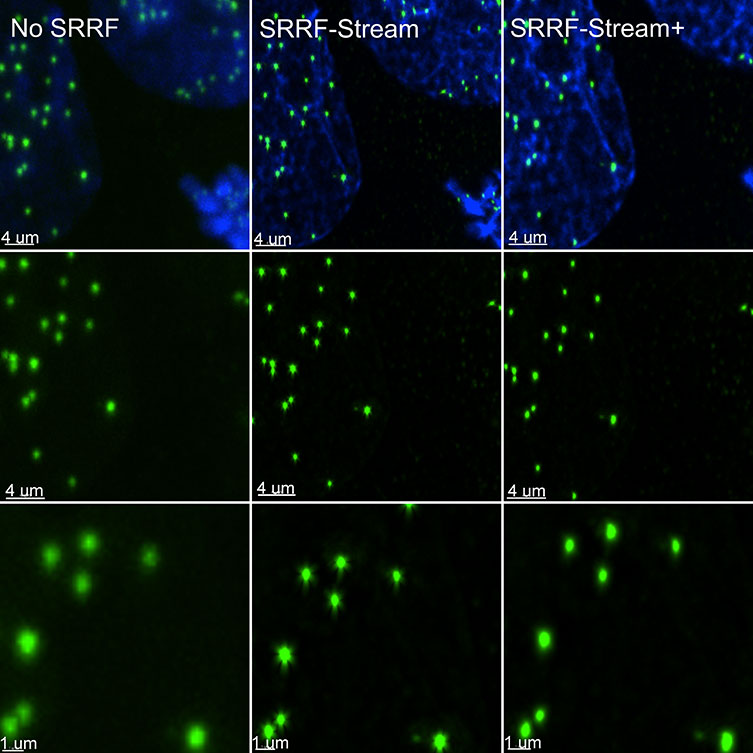
Figure 7 – SRRF stream+ delivers high-quality data. At very high magnifications, in specific structures, such has kinetochores some Star artefact could occasionally appear. SRRF-Stream+ radiality computation in 24 directions eliminates the star artefacts. Hela cells stained for Cenp-A (green-Kinetochore) and DAPI (blue-DNA) were imaged in the Dragonfly with an Ixon888 Ultra camera.
As for Andor original SRRF-Stream advantages, they are maintained in SRRF-Stream+ but with the improved image quality. Super-resolution imaging processing is still carried out at ultra-fast processing rates (up to 30X faster) than ImageJ SRRF (“NanoJ-SRRF’), and SRRF image acquisition/processing is captured on the fly, in parallel to the acquisition of the data. SRRF-Stream+ algorithm delivers images with a final resolution between 50-150 nm and due to its low energy requirements is an ideal solution for live-cell microscopy. Super resolved images can be acquired using with large fields of view, and importantly super-resolution microscopy is not restricted to cell surface events. With SRRF-Stream+, it´s possible to acquire a super-resolved image deep inside cell or tissues, and there is no need for special sample preparation.
Since it’s launch, SRRF-Stream has become widely used for many applications. Examples of using SRRF-Stream in iXon cameras include: to analyse mitochondria trafficking into cell terminus via microtubule destabilisation (5), or analysis of HIF1α nuclear translocation in mesenchymal stem cells (6). The Dragonfly multimodal confocal system was also be used to acquire SRRF-Stream images, examples are: analysis of cytokinesis using optogenetic tools (7) as well as vesicle trafficking studies on analysing the effects of class II PI3Ks in the regulation of clathrin-dependent pinocytosis (8). Other applications of SRRF-Stream+ include analysis of protein structure at a sub-organelle level, tracking of single molecules inside cells, membrane fusion studies of individual SNARE protein machinery, intracellular skeleton reassembly (changes to actin fibre meshwork).
In conclusion, we have improved our SRRF-Stream algorithm delivering live-cell compatible super-resolution with even better results than before. SRRF-Stream+ delivers super-resolved images of intracellular structures without artefacts, can compensate for the effects of cameras fixed pattern noise, and delivers high-quality super-resolved images.
Many other applications and discoveries are waiting to be revealed using SRRF-Stream+. Do you want to know more about SRRF-Steam+? Contact us we will be delighted to show it in action.
[1] For more detailed information on how to sample on iXon cameras please go to Andor original SRRF-stream tech note.
[2] Full field of view (1024 X1024) images acquired with Ixon Ultra.
