Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
The interaction of electromagnetic waves with matter is described by the index of refraction n(r) =1- δ(r)+iβ(r), where δ(r) describes the phase shift and β(r) the change in absorption. For soft tissue, δ(r) is up to three orders of magnitude larger than β(r) [1]. Therefore, image contrast for low absorbing samples can be enhanced by considering the phase contrast occurring from the phase shifts. One method to visualize phase contrast is propagation-based imaging (PBI). The phase shifts are converted into measurable intensities, due to self-interference of a coherent X-ray beam in the free space between sample and detector. Phase retrieval algorithms allow the reconstruction of the phase shifts in the sample plane from the intensities in the detector plane, which need to be recorded with a camera of small pixel size and sufficient frame rates in order to reduce the duration of a tomographic scan.
The Zyla-HF sCMOS (scientific CMOS) camera (model Zyla-5.5X-FO from Andor Technology with a 10-tap CameraLink frame grabber card) was integrated into the synchrotron endstation „GINIX“ (Göttingen Instrument for Nano-Imaging with X-rays) [2] at the coherence beamline P10 of the PETRA III storage ring at DESY. The monochromatic X-ray beam was prefocussed with a KB-System. An X-ray waveguide was placed into the X-ray focus to downsize the spot size, smooth the beam profile and increase the coherence. Distance between X-ray focus, sample and detector determined the geometrical magnification M, which was between 40 and 130 for high resolution tomograms. For M=40, this lead to an effective pixel size of 0.16 μm. For unstained biological samples with a thickness of 1 mm, an X-ray energy of 8 keV was used, while 13.8 keV was used for 1 mm heavy metal stained samples. For these X-ray energies, the photon yield was substantially higher with a 15 μm thick GADOX (Gd2O2S:Tb) scintillator compared to a 20 μm YAG:Ce scintillator.
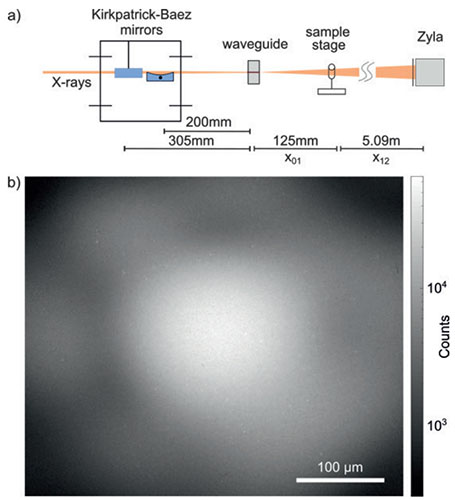
Fig.1 (a) Synchrotron setup. Monochromatic X-rays are focused by a system of KB-mirrors. An X-ray waveguide is placed in the focal plane, followed by the sample stage and the Zyla-HF detector. (b) Empty-beam image of the waveguide illumination in 5.09 m distance with 350 ms exposure time shows the granular structure of the 15 µm thick GADOX scintillator
We demonstrate the setup performance for the soft tissue of a murine heart [3]. The spatial organization of cardiac muscle tissue exhibits a complex structure on multiple length scales, from the sarcomeric unit to the whole organ. In order to image subcellular features of the cardiomyocytes, we performed x-ray phase-contrast tomography experiments. Fig. 2 shows a typical projection (a) and the corresponding phase reconstruction (b). Further, a slice of the 3d volume showing the quality of the data (c). Sub-cellular structures such as nuclei, erythrocytes and myofibrills can be identified. In the given slice, also a blood vessel with the surrounding endothelial cells is visible. In further analysis this data was used to extract the local sarcomeric periodicity in the cardiac tissue. Future extension of this work will be directed at bridging the gap between structure and function, also in view to increase the understanding of cardiovascular diseases.
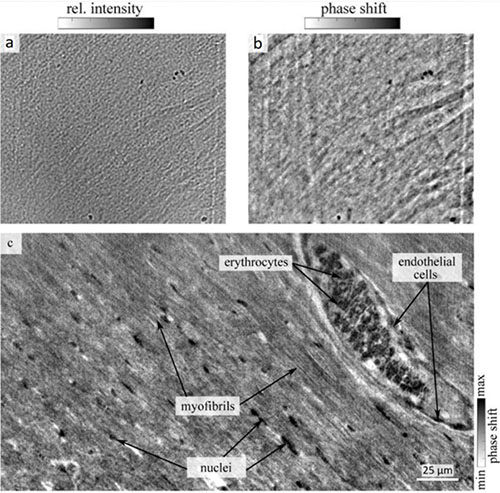
Fig.2: (a) Empty-beam and dark-field corrected projection of a 1 mm biopsy of a murine heart embedded in paraffin. (b) Phase reconstruction of the projection using a nonlinear-Tikhonov approach of the CTF. (c) Reconstructed tomographic slice through the 3d volume of the cardiac tissue. Sub-cellular features such as myofibrils, vessels filled with erythrocytes, endothelial cells surrounding the vessels, and nuclei in between myofibrils are visible.
The Zyla-5.5X-FO sCMOS camera was controlled by the μManager software v.1.4.23 [4] without any constraints in handling. Images were acquired in the 16-bit mode with a mean baseline offset of 100 adu. Resolution was measured at a rotating copper anode laboratory source (20 kV/10 mA). The MTF derived from the slanted edge method showed 40 cy/mm at the 10% crossing.
For high-resolution propagation-based phase-contrast imaging (PBI), an X-ray sensitive and high resolving detector with low read-out noise is preferable. We performed PBI experiments at the synchrotron endstation „GINIX“ with the Zyla-5.5X-FO sCMOS camera at 8 keV and 13.8 keV X-ray energy. To increase the detector sensitivity at these energies, we used a 15 μm thick GADOX instead of a 20 μm YAG:Ce scintillator. Image quality in terms of resolution and sensitivity was convincing, so we are fully satisfied with the performance of the camera, which fulfilled all our requirements.
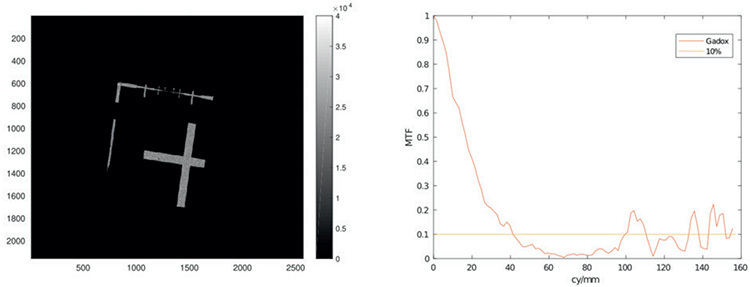
Fig.3: (a) Image of the slanted edge object (LIGA) with 3 s exposure time. (b) MTF obtained from fig (a). 10% crossing at 40cy/mm.
Jasper Frohn
Research group “Structure of biomolecular assemblies and x-ray physics”
Institute for X-ray Physics
Georg-August University Göttingen
Friedrich-Hund-Platz 1
37077 Göttingen
Germany
Phone: +49 (551) 39-25066
E-mail: jasper.frohn@phys.uni-goettingen.de
Web: http://www.uni-goettingen.de/en/563229.html
Date: September 2022
Author: Jasper Frohn (Georg-August University Göttingen)
Category: Application Note
