Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
All organisms from basic single-cell organisms through to complex multicellular organisms, including humans, are subject to a 24-hour cycle due to the rotation of the earth. Organisms have evolved a specific biological clock system, often called the Circadian Clock to synchronise with this cycle. Since the circadian clock has been found to play a key role in many important biological functions, research into the underlying regulatory processes are becoming of increasing interest.
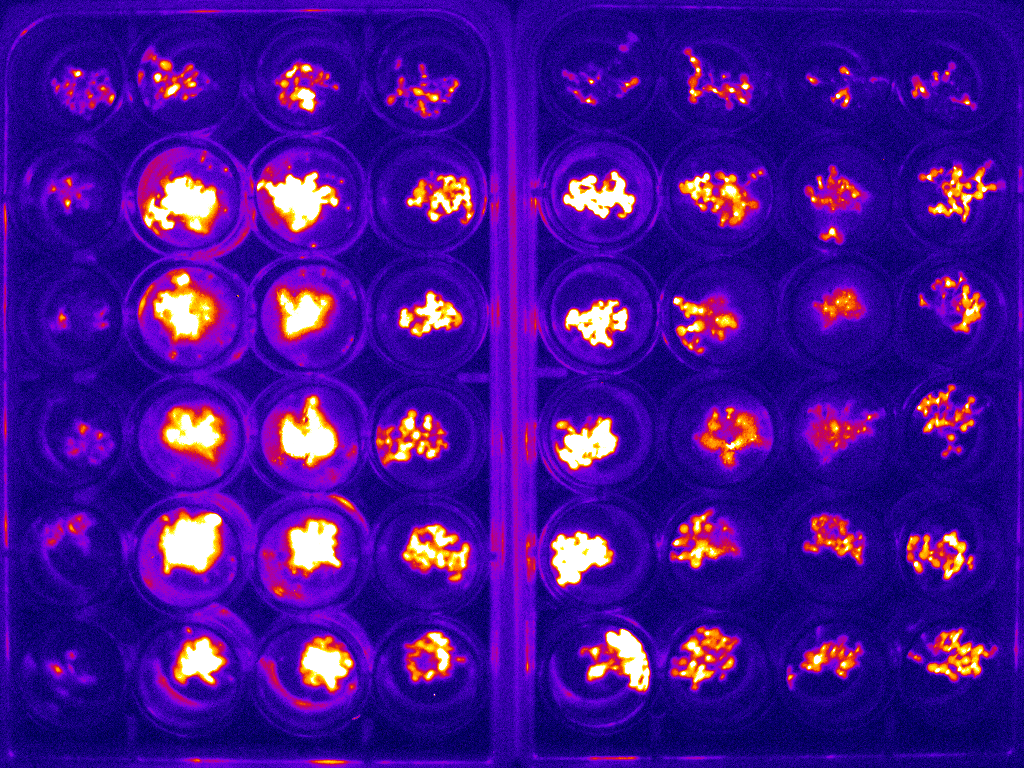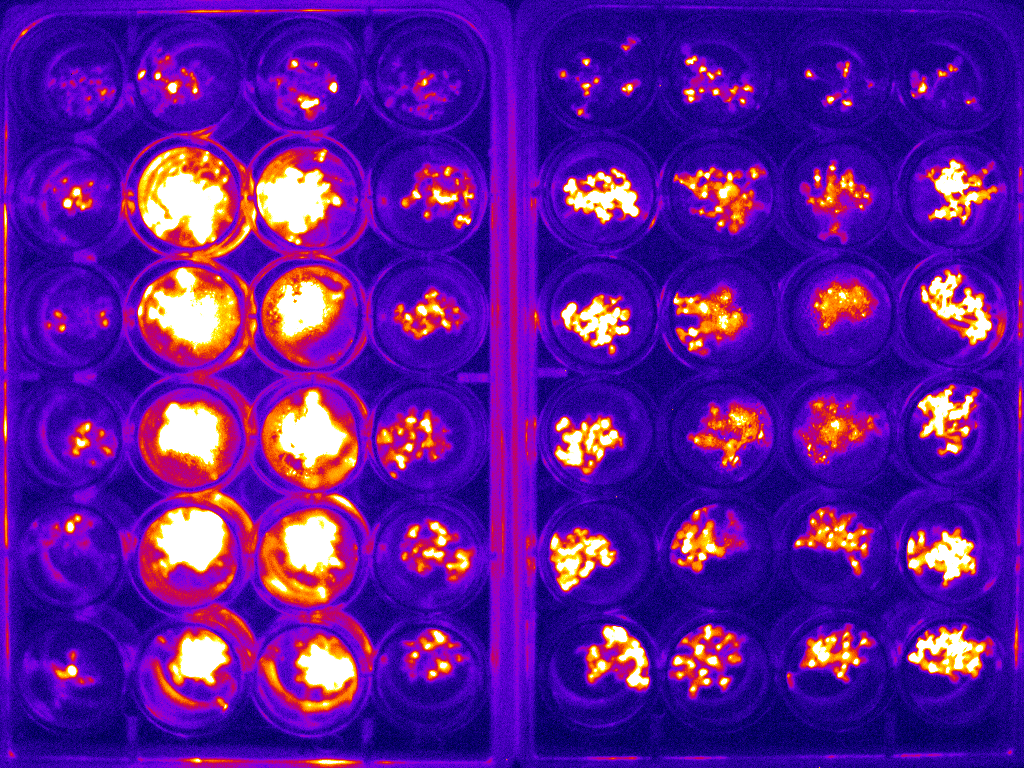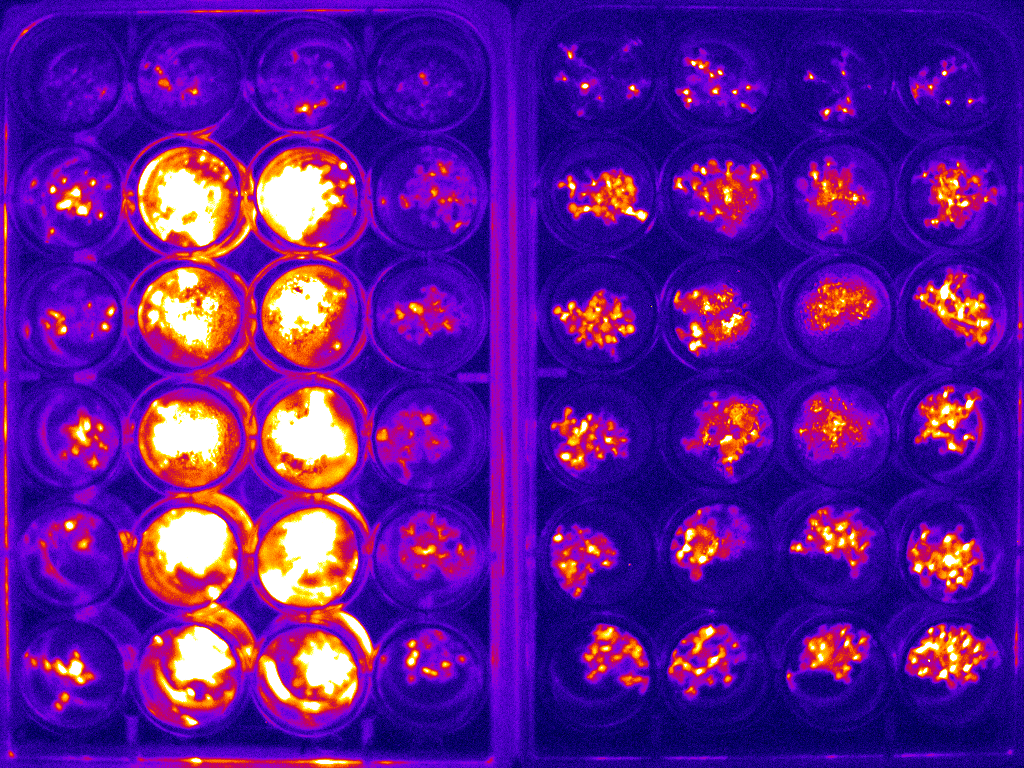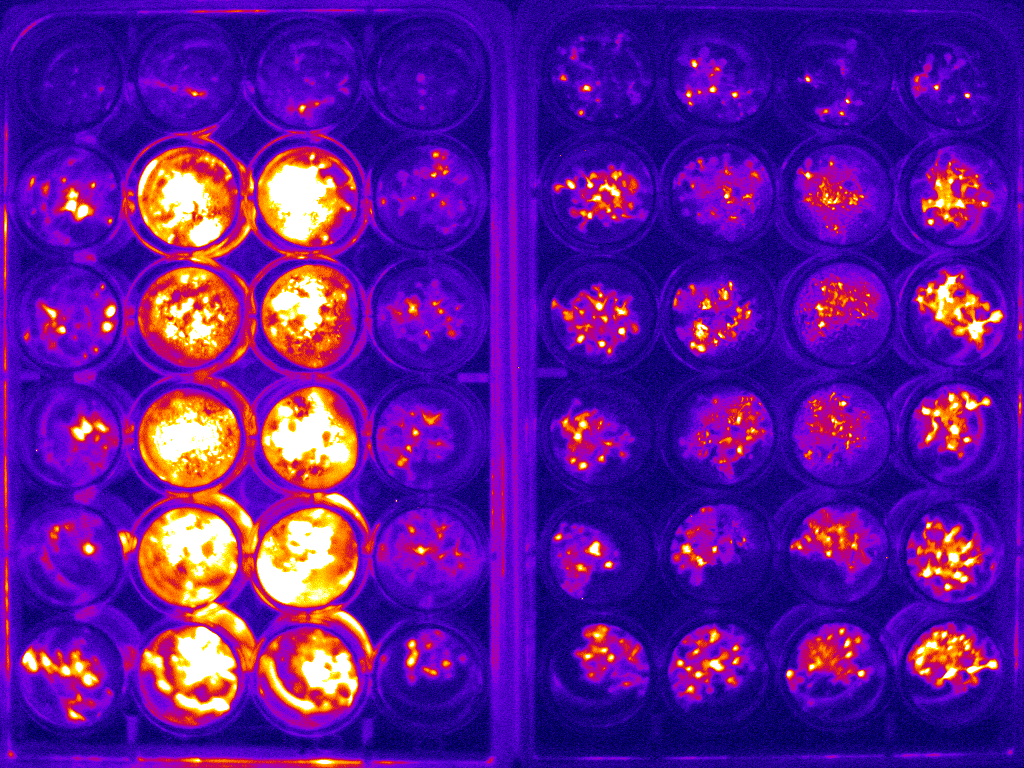
Example of bioluminescence timelapse of Arabidopsis to investigate the circadian clock.
The biological clock system can be divided into three functional parts:
Professor Liu (Liu Lab, Shanghai Institutes for Biological Sciences, CAS, Shanghai), focuses on the study of the mechanism of light regulated transcription, and light regulated development using Arabidopsis as a model organism. They also work on the molecular mechanism of light regulated transcription and circadian clock regulated transcription.
In order to perform research in this area, Professor Liu’s group have built a complete bioluminescence detection system that enables the precise control of light and temperature levels, this is shown in figure 1. In this system, the output signal is measured using the bioluminescence signal from specifically labelling with Luc protein in Arabidopsis grown in a 24-well plate. A high sensitivity camera equipped with a commercial lens is then used to record the image.

Figure 1: The Bioluminescence detection system used by the Liu Lab features precise control of light and temperature and high performance CCD imaging camera.
An example image for two 24-well plates is shown in figure 2(a). The whole bioluminescence image of the Arabidopsis in the two sets of 24-well plates was captured simultaneously. The average of the signal was used to determine the expression of the specific protein of interest. This experiment was carried out at 1 hour intervals over the course of 6 days. The circadian rhythm curve generated from this is shown in figure 2(b).
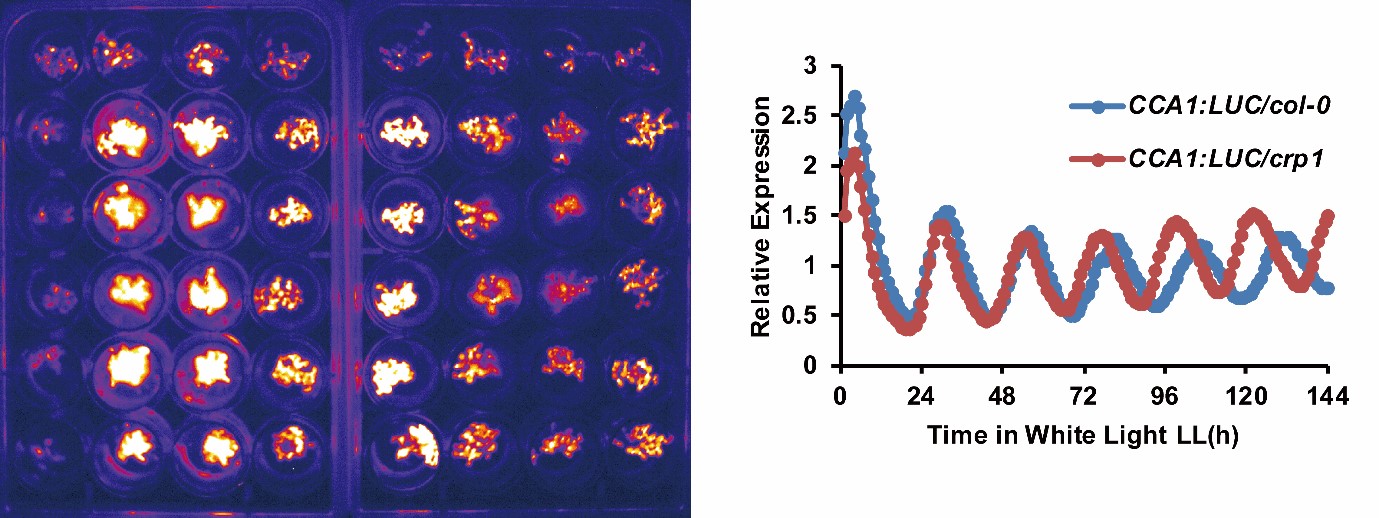
Figure 2: (a) typical bioluminescence image of Arabidopsis imaged in two sets of 24-well plate;(b) typical circadian rhythm curve
Due to the very low signal levels that are produced and the long duration of the experiments the detector choice is critcal to peforming such studies effectively. When compared against the traditional leaf movement analysis method, there are two advantages (data available in reference 5):
The iKon-M CCD camera was found to be the ideal solution for this work. The iKon-M features a high sensivity sensor with 95% QE. Very low dark current means that there is a very low noise floor. This combination is important since it means that the signals can be detected against the background noise over the long timescales these experiments require. The 13 mm x 13 mm sensor size of the iKon-M is perfectly matched to capturing two sets of 24-well plate in high resolution making for efficent collection of experimental data.
"The iKon-M CCD camera helps us obtain data more accurately and more conveniently. We can also do other important scientific research using protein interaction based BiFC and BiLC methods."
- Libang Ma, Liu Lab
All data courtesy of the Liu Lab. Find out more about the exciting research at the Liu Lab here.
Date: September 2019
Author: Wang Kun and The Liu Lab, Shanghai Institutes for Biological Sciences, CAS, Shanghai
Category: Case Study
