Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
For conventional imaging, illumination of the specimen is something we try to carefully control. While we need illumination to observe the sample, too much light can have adverse effects on the very biology we are trying to understand. In fluorescence microscopy, repeated excitation of a fluorophore can result in its transition from the excited singlet state, to an excited triplet state. This results in an irreversible chemical modification that prevents further fluorescence from occurring. This is called photobleaching and generally something to avoid in normal imaging by using low illumination and high sensitivity detectors. However, it is possible to exploit this condition to make many useful measurements of processes of the cell that would otherwise by very difficult to study by other means. Examples of how photobleaching may be applied include:
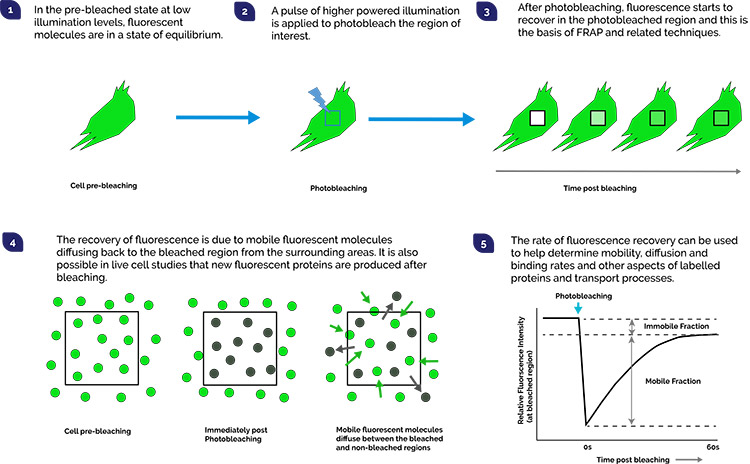
To apply photobleaching techniques successfully we need to have fine control over illumination delivery parameters: the required wavelength, a sufficient illumination intensity, and timing of this to the region of interest. Andor’s MicroPoint and Mosaic are two components that can be integrated with your microscope, or with a Dragonfly Confocal System to great effect.
MicroPoint is a pulsed nitrogen pumped tunable dye laser system. This makes it ideal for the following application requirements:
Mosaic is a digital mirror-based array (DMD) that can be combined with light sources from LED, arc lamps to higher power lasers including MicroPoint.
Date: February 2023
Author: Dr Alan Mullan
Category: Solution Note
