Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
In the Kucenas Lab, we are fascinated by the cellular and molecular mechanisms that drive glial development. Using zebrafish (Danio rerio), coupled with genetic and pharmacological perturbation, single cell manipulation, laser ablation, small molecule screening, single-cell RNA-sequencing (scRNAseq), and in vivo, time-lapse imaging, we have discovered novel glial populations, revealed how glia communicate in several unique contexts, and created many new tools for our studies and the broader community. Recently, we have become very interested in the compensatory behaviors of professional and non-professional phagocytes after injury and in disease and use zebrafish as a system to track the dynamic interactions and behaviors of glia in these scenarios.
In order to study the role of phagocytes after injury, we need an imaging system that allows us to precisely ablate single cells or transect axons while leaving the rest of the environment unperturbed. Andor’s MicroPoint nitrogen-pulsed dye laser achieves this level of precision and can be focused, for example, on individual neurons deep in the spinal cord of a developing zebrafish larva. One area that we have become interested in exploring is how microglia, which are macrophage-like CNS resident phagocytes, respond to injuries or dying neurons in the brain and spinal cord. Broad ablation techniques that eliminate entire populations of cells cause significant inflammation and can ultimately lead to larval death. In order to investigate the precise cellular and molecular mechanisms used by microglia to respond to the death of a single neuron, we mounted Tg(nbt:dsred;mpeg1:egfp) larval zebrafish, which express cytoplasmic DsRed in neurons and cytoplasmic GFP in microglia, in low-melting point agarose in a glass bottom Petri dish. Using a MicroPoint laser mounted on a Dragonfly spinning disk imaging system, we then ablated a single nbt+ spinal cord neuron while leaving neighboring cells intact. To investigate how local mpeg1+ microglia respond to this perturbation, we used the Dragonfly to rapidly collect multi-channel z-stack time-lapse images that captured microglial dynamics immediately after the injury. The image file was then imported into Imaris for 3D rendering and analysis.
From this experiment we were able to visualize a GFP+ microglial cell rapidly respond to the loss of a single neuron in the developing spinal cord (Figure 1 & Movie 1). This is important because it shows how we are able to specifically control the accuracy of the laser ablation and then assess and quantify cell behaviors during an injury response. To extend these studies and fully utilize this system, we are now using genetic and pharmacological methods to perturb microglial function to study how this affects the injury response in the ablated cell microenvironment. Other interests in the lab involve studying how peripheral glia regulate injury repair following nerve transection in the PNS and using single oligodendrocyte ablations to study the mechanisms that regulate oligodendrocyte tiling and remyelination in the CNS. Being able to couple precise cellular ablation with high resolution, in vivo, time-lapse imaging, we are able to ask questions that are simply impossible to address using other systems.
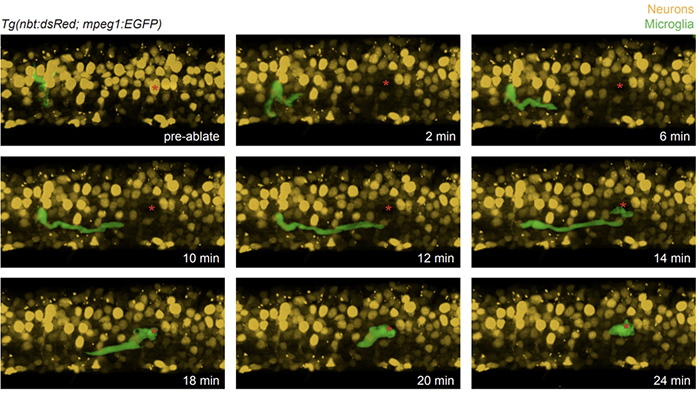
Figure 1. Microglia respond to targeted ablation of a single neuron in the developing spinal cord. Stills taken from an in vivo, time-lapse movie of a Tg(nbt:dsred;mpeg1:egfp) larva where neurons express DsRed (yellow) and microglia express GFP (green). Using the MicroPoint ablation laser, we targeted a single neuron (red asterisk) for ablation while maintaining the integrity of the spinal cord and not disturbing neighboring cells. Within two minutes, the microglia present near the site of ablation begins to respond and by 12 minutes post ablation, arrives at the site of where the ablated neuron was. This shows the requirement and ability to specifically control the accuracy of laser ablation to then assess and quantify cell behaviors during an injury response.
Movie 1. Microglia respond to targeted ablation of a single neuron in the developing spinal cord. In vivo, time-lapse movie of a Tg(nbt:dsred;mpeg1:egfp) larva where neurons express DsRed (yellow) and microglia express GFP (green). The ability to capture, in real time, the dynamic responses of microglia to injury in the developing spinal cord, allows us to ask questions about the nature of the cell behaviors microglia use when responding to dead or injured tissue.
Date: September 2025
Author: Andrew Latimer and Sarah Kucenas, Department of Biology & Program in Fundamental Neuroscience, University of Virginia Charlottesville
Category: Application Note
