Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Optogenetics combines optics and genetic manipulation techniques to allow for very precise control of processes within cells using light. With an interconnected network of billions of neurons in the brain it is very difficult to unravel and study what is happening in psychiatric and neurodegenerative disorders. Conventional tools for study of neurons involving simulation by electrodes or various drugs are very useful tools, but they do not allow modulation of the activity of individual neurons. This limits how neural pathways of interest can be mapped and their function determined as part of that wider network. Key to optogenetics studies are opsin genes which act like “light sensors”. Opsins respond to light, and by genetically modifying neurons to include opsin genes it is therefore possible to use light to open the ion channels of a single neuron and fire the electrical signal.
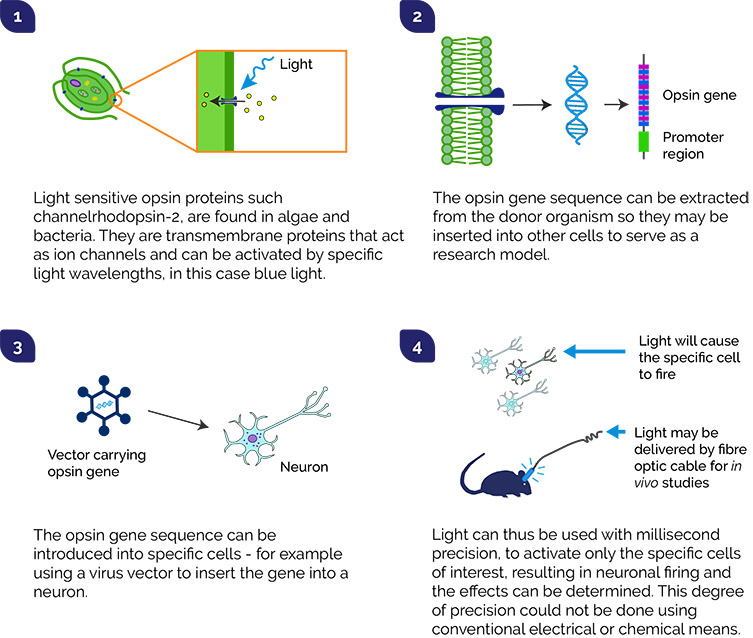
One of the key papers was that of Boyden and Deisseroth (2005) who reported an effective method to excite neurons in rats using the opsin channelrhodopsin (derived from green algae). In the following years, many research groups have gone on to expand what is possible by optogenetics e.g. by identifying and modifying opsins that respond to different wavelengths, and to be able to switch off neurons. Areas that have benefitted from these developments are far reaching, from how memories are formed, addiction studies, providing better management of Parkinson’s disease tremors using deep brain stimulation, to restoring vision
There are many supporting technologies that have helped enable this technique to become established: delivering specific wavelengths of light to opsin expressing cells with precise timing, controlling illumination through devices such as Mosaic, while sCMOS cameras are often the most suitable detector solution for these experiments, e.g. when high frame rates required for calcium imaging.
References
Date: February 2020
Author: Dr Alan Mullan and Dr Claudia Florindo
Category: Application Note
