Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Plant responses to abiotic stress are dynamic and complex, and their study is important not only to understand how plants react to the environment, but also to provide additional resources for crop improvement research in the future.
Imaging plants is complex, and several technical challenges must be overcome such as autofluorescence, tissue penetration, speed of acquisition. The ideal system for plant imaging will allow researchers to gain insights in plant biology as well as create tools to improve stress resistance in important crops.
In this article we present:
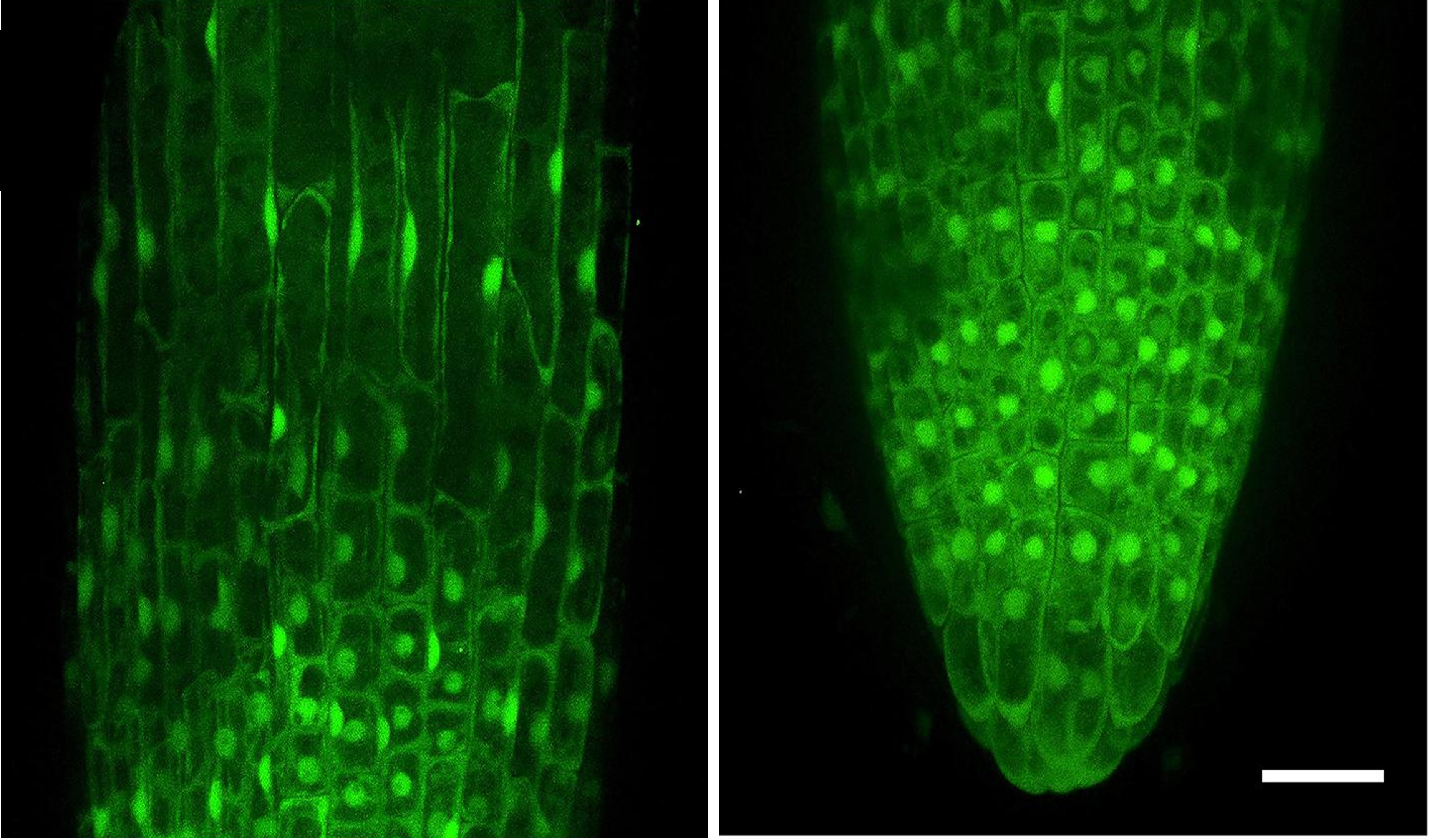
Figure 1. Representative images showing localization of overexpressed NMig1 gene in the primary root meristem of 6-day-old Arabidopsis thaliana. Image was acquired with Andor Dragonfly confocal imaging system. Scale bar 20um (Velinov V et al. Front. Plant Sci, 2020, 11, 815.)
Modern plant sciences require the use of various experimental methods, and light microscopy plays a significant role among them. As plant imaging is typically performed on different scale levels, it is crucial to use a microscope that quickly delivers excellent quality images from sub-cellular structures to large organs.
Andor Dragonfly, a high-speed multi-point confocal system, offers unique features that are ideal for plant imaging. Its high speed of acquisition and real-time 3D rendering allows for rapid imaging and visualization without any loss in resolution. The patented Borealis uniform illumination, paired with a large field of view and seamless stitching are the perfect tools to image large tissues in just a matter of minutes. The system also provides the use of infrared lasers, which are great for deep imaging and reduce the risk of autofluorescence in plants.

Figure 2. Prof Valya Vassileva using Andor Dragonfly in the quest to find new molecular regulators that improve crop resistance to stress.
During their life cycle, all plants are subjected to a variety of environmental stresses, such as soil salinity, drought, and extreme temperatures. These conditions, also called abiotic stresses, can lead to abnormalities in cellular homeostasis, which can have detrimental effects on plant growth and development.
To combat such stresses, plants have developed a variety of complex and well-coordinated responses that include changes in plant metabolism and adjustments in their architecture and morphology. At a molecular level, plant cells undergo changes in gene expression that are triggered by internal messengers, like hormones and free radicals, or by other molecular mechanisms (e.g., epigenetic changes).
As mentioned above, plant responses to abiotic stress are dynamic and complex.
Research on this topic is therefore significant because it allows one to:
| Glossary | |
| Abiotic Stress | Negative impact of non-living factors on the living organisms, such as plants. |
| Auxins | Auxins are a class of plant hormones (or plant growth regulations) |
| Ethylene | Ethylene is an unsaturated hydrocarbon gas acting naturally as a plant hormone. It is one of the major hormones involved in the stress response in plants. |
| Gravitropic response | Growth or movement of a sessile organism in response to gravity, as the downward growth of plant roots. |
| Homeostasis | State of steady internal, physical, and chemical conditions maintained by living systems. |
| Meristem | Part of the plant that contains undifferentiated cells (meristematic cells) capable of cell division. |
| NMig1 | Nuclear Migration 1 gene; a newly discovered Arabidopsis gene with potential regulatory function in root development and tolerance to abiotic stress. |
| NudC | Nuclear distribution gene C: a highly conserved gene implicated in essential cellular processes |
| R2D2 | A highly sensitive, rationmetric sensor that detects auxin |
Prof Vassileva’s research aims to understand the molecular mechanisms of plant resistance to stress. Below we present an overview of two plant biology research projects in her laboratory at the Institute of Plant Physiology and Genetics at the Bulgarian Academy of Sciences.
In a recently published article from the “REGULATION OF THE GENE EXPRESSION” lab, a new gene with a previously undescribed and significant function in root development and abiotic stress tolerance in Arabidopsis thaliana has been reported (Velinov et al. 2020). The gene belongs to the NudC gene family, and it was named NMig1 for Nuclear Migration 1.
In order to understand the function of this previously uncharacterized gene, the group took advantage of the Andor Dragonfly multimodal confocal system to study the expression pattern of NMig1 in transgenic lines that overexpress the fusion protein GFP-NMig1.
The confocal microscopy images show a very strong GFP fluorescence detected in the primary roots, with the most intense signal in the root apical meristem. The observed signal in the overexpressing transgenic line was associated with enhanced root growth and branching under several abiotic stress factors when compared to a wild type.
Very importantly the lab realized that:
“These results could contribute to breeding more resilient crops with improved root architecture under adverse environments.”
To have an overarching understanding of plant resistance to abiotic stress the laboratory has focused on the synergy between ethylene and auxin hormones to stress regulation.
Auxins play a cardinal role in the coordination of many growth processes in plant life cycles and are essential for plant body development. Ethylene is another plant hormone involved in the stress response
The researchers took advantage of a highly sensitive ratiometric auxin sensor R2D2 developed by Liao et al. (2015). The R2D2 sensor allows high-definition evaluation of changes in active auxin levels in distinct cell files: the ndtTomato signal (magenta) is not sensitive to auxin levels and is used as control, while the Venus signal (green) decreases when the auxin levels rise.
This high-resolution, fast evaluation of variation in hormone levels was done using Andor Dragonfly spinning disk confocal microscope.
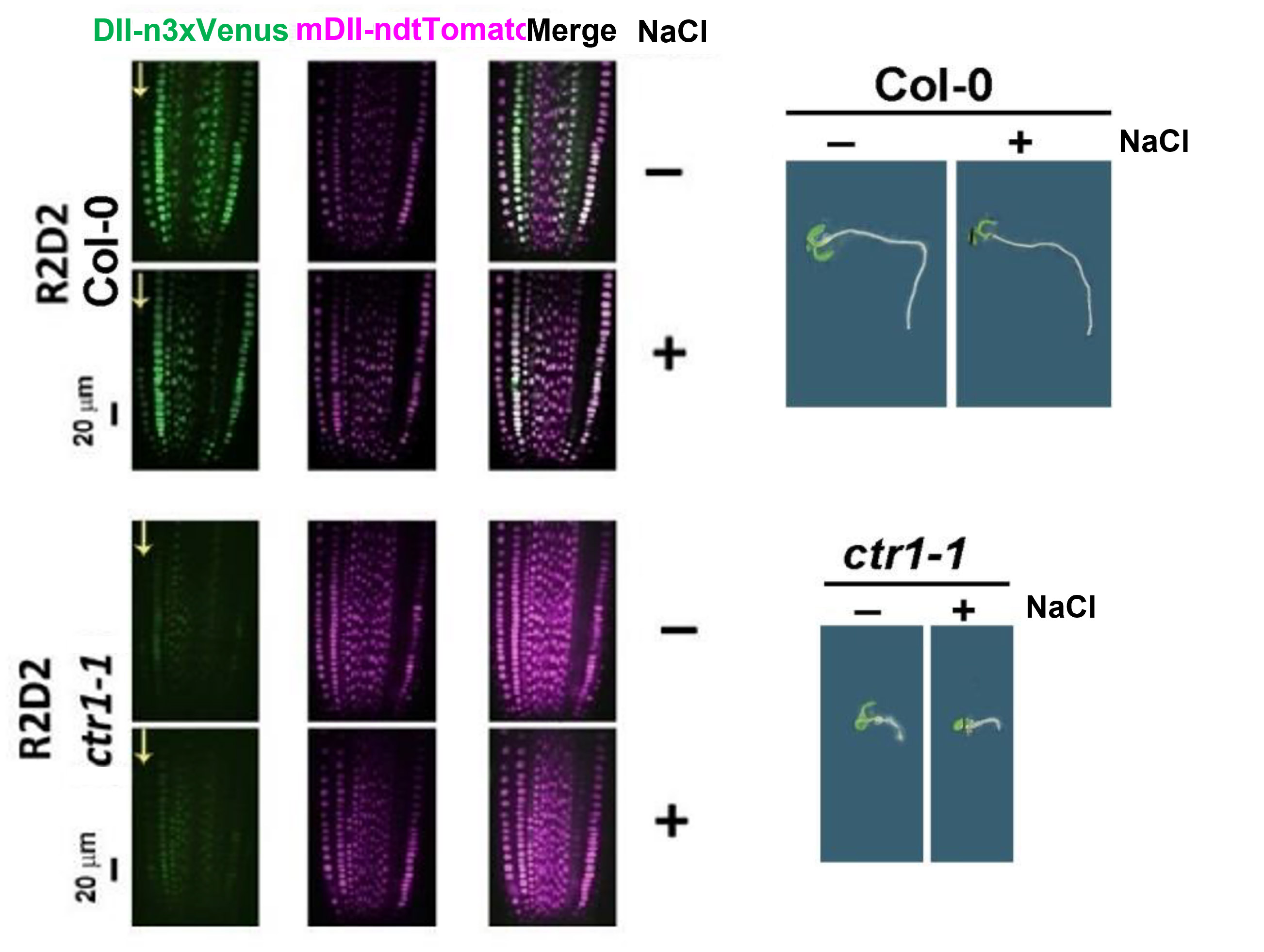
Figure 3. Fluorescent signal acquired with Andor Dragonfly confocal imaging system. Ratiometric evaluation of R2D2 in roots of control and 100 mM NaCl-treated (48 h) wild-type plants and their hormozygous F3 cross with ctr1-1. Arrows mark the epidermal cell file. (Vaseva et al. Plants, 2021, 10(3): 452.)
With the aim to test plant resistance to saline conditions, Dr. Vaseva and colleagues introduced the R2D2 sensor to monitor auxin levels into control plants and ethylene signaling mutants (Vaseva et al. 2021). The analysis was done using the Dragonfly confocal spinning disk microscope to monitor the changes in the fluorescent signal of the auxin reporter (R2D2).
The Dragonfly was the ideal tool to analyze the plant response to stress, because of its high background rejection, large field of view, and excellent sensitivity. Significantly, since the plant responses to stress are tissue and even cell-specific, it was critical for Dr Vaseva to localize the auxin signal at a single-cell level.
“The confocal observations obtained with the Dragonfly confocal system allowed us to map the active auxin increase to specific root epidermal cells, a phenomenon linked to the root growth inhibition provoked by the stress”.
The results have revealed increased auxin activity provoked by the salt stress in control plants, accompanied by a loss of gravitropic response. However, the constitutive ethylene signaling mutants (ctr1-1) always had higher basal auxin levels (represented by a low green signal in Figure 2) and showed no significant alterations in gravitropic bending upon salt stress. Overall, these results suggest that ethylene signaling could be involved in the plant’s response to salt stress through the regulation of local auxin availability.
In conclusion, the researchers expect that the results are likely to find an application in novel strategies for the improvement of stress tolerance of economically important crops.
The research projects of Prof. Vassileva’s lab are focused on elucidating the molecular mechanisms of plant adaptation to abiotic stress.
In order to achieve such goals, the laboratory studies genetic and epigenetic aspects of gene expression regulation. The laboratory members use the model plant species Arabidopsis thaliana and Lotus japonicus, and crop plants, such as Triticum aestivum, Zea mays, and Phaseolus vulgaris to address the resistance to abiotic stress. The laboratory generates and characterizes transgenic plant lines where the genes of interest are overexpressed or silenced.
With an experimental approach that branches from genetics to biochemistry, and finds support on advanced light microscopy approaches, the lab aims at:
References
Herud-Sikimić, O., Stiel, A.C., Kolb, M. et al. A biosensor for the direct visualization of auxin. Nature 592, 768–772 (2021). https://doi.org/10.1038/s41586-021-03425-2
Liao, CY., Smet, W., Brunoud, G. et al. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods 12, 207–210 (2015). https://doi.org/10.1038/nmeth.3279
Vaseva II, Mishev K, Depaepe T, Vassileva V, Van Der Straeten D. The diverse salt-stress response of Arabidopsis ctr1-1 and ein2-1 ethylene signaling mutants is linked to altered root auxin homeostasis. Plants, 2021, 10(3): 452. https://doi.org/10.3390/plants10030452
Velinov V, Vaseva I, Zehirov G, Zhiponova M, Georgieva M, Vangheluwe N, Beeckman T, Vassileva V. Overexpression of the NMig1 gene encoding a NudC domain protein enhances root growth and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci., 2020, 11, 815. https://doi.org/10.3389/fpls.2020.00815
Author: Dr Anna Fasoli, Dr Claudia Florindo, Dr Irina Vaseva, Dr Valya Vassileva
Category: Application Note
