Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
BC43 allows all laboratories in biomedical sciences, industry or elsewhere, access to an easy-to-use high quality multimodal confocal imaging system to suit their imaging needs. BC43 is a Benchtop Confocal imager that includes 4 lasers and 3 imaging modalities:

Figure 1 - Image of BC43 Multimodal Confocal Microscopy
In this article we will explain the features of Andor Benchtop Confocal - BC43, including the imaging modalities, software and hardware features. Finally we will look at an overview of the some of the typical applications that the BC43 is suited for. Click on the index below to navigate to a specific section.
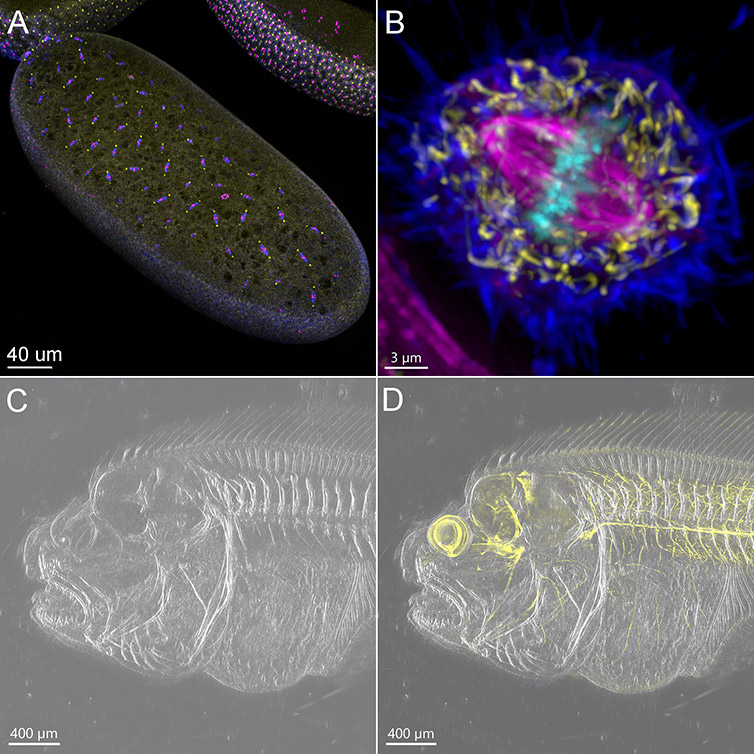
Figure 2 - BC43 is a Multimodal Imaging System. This figure shows pictures acquired using different imaging modalities of BC43.
A) Confocal Imaging modality was used to acquire an early-stage Drosophila Embryo. The image presented is a result of the stitching of 6 imaging fields, over a Z range of 50 µm Blue-Microtubules, Yellow-centrosomes, Magenta-DNA. Image credits Ines Baião-Santos, Álvaro Tavares – Universidade do Algarve, Claudia Florindo - Andor Technology. B) Widefield image of a mitotic cell. Image was acquired in widefield mode with the deconvolution option activated in the protocol. Dark blue – actin, yellow - mitochondria, magenta - microtubules, cyan-DNA. Image credits Claudia Florindo Andor Technology. C) DPC image of Flat-fish head D) Merge Confocal and DPC image of flat fish head. C & D Images are composed of 10 fields acquired with the DPC imaging option (C), and DPC merged with confocal imaging option (D). 395 - Z slices were acquired for each channel covering a range of 632 µm. Sample was cleared and stained with acetylated tubulin (yellow). Image credits Marco Campinho – Universidade do Algarve, Claudia Florindo – Andor Technology.
With Andor Benchtop Confocal - BC43, there is no need for a dark room, you can have BC43 on the bench side with your experiments.
| Benefits | Features | |
| Hardware | Bench Top Microscope | 1. Integrated dark box and small footprint 2. Easy control with ergonomic joystick 3. Built in anti-vibration mechanism |
| Quick Sample Overview | 1. Extremely user friendly 2. 2x objective to quickly locate and scan the sample 3. Quick montage for very large samples |
|
| Multiple Acquisition Options | 1. XYZ motorised high precision stage 2. 4 laser lines 3. 10 predefined acquisition channels 4. Fast confocal & Widefield imaging |
|
| Highly Sensitive sCMOS Detector | 1. Large field of view (18.4 mm) 2. High sensitivity sCMOS detector with 2000x2048 resolution (4 Mega Pixel) 3. 16-bit dynamic range |
|
| Seamless Stitching & Accurate Image Quantification | 1. Andor Patented Borealis Uniform Illumination | |
| Software | Start Imaging Quickly | 1. Super-fast learning curve (Intuitive) 2. Clear, logical workflow 3. Point & click to centre image |
| Find the Sample and Maintain Focus | 1. Andor patented Focus Seek & Lock 2. Time lapse imaging |
|
| In line Image Processing | 1. GPU Accelerated Deconvolution 2. Stitching 3. Real time 3D rendering |
Table 1 - Hardware & Software Advantages of Andor Benchtop Confocal - BC43
BC43 includes 3 imaging modalities and can be used as:
For an overview of imaging modalities, and their respective strengths please go to Overview of Microscopy Techniques for Life Science
These imaging modalities can be combined as needed for users' experimental requirements. For example, it can be extremely useful to combine confocal imaging with the transmitted light technique DPC, for an overview of the full sample and where the fluorescence is localised in the context of the organism.
Andor Benchtop Confocal's most significant imaging modality is multipoint confocal. With confocal imaging in BC43, Andor allows the scientific community access to one-click 3D microscopy, which is simple, high quality and affordable.
The confocal modality of BC43 is delivered by a dual micro lens spinning disk integrated with Andor's patented Borealis uniform illumination and a high sensitivity sCMOS detector.
The 50 µm pinhole and optimised pinhole spacing deliver exceptional background rejection whilst allowing imaging deep into thick specimens. The BC43 delivers excellent results even in samples with thicknesses of over 500 µm. (as shown in the cleared flatfish in figure 3).
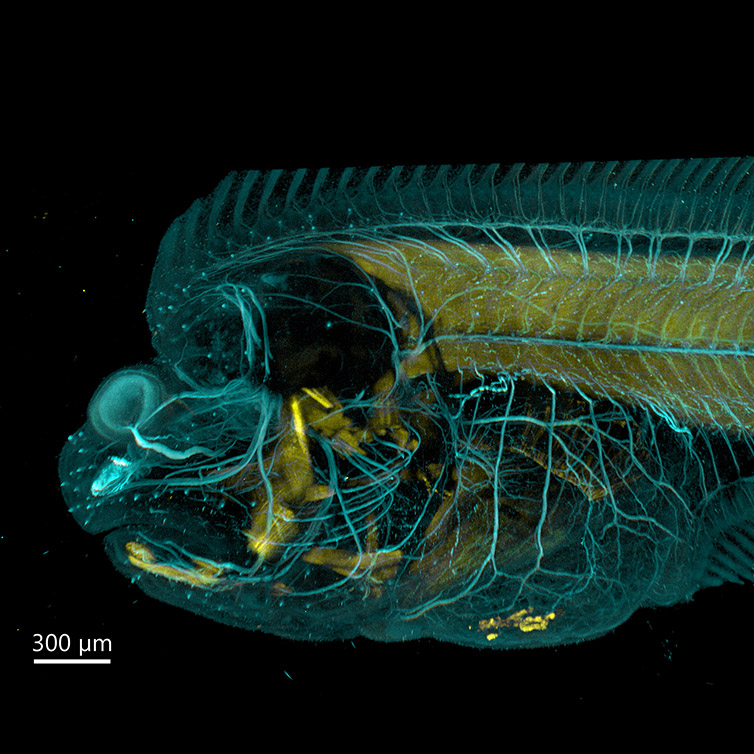
Figure 3 - Flat Fish Imaged With BC43 using Confocal Option. Image acquired with Andor Bench Top Confocal – BC43 using multiple tile acquisition and montage. 6 tiles were acquired to compose the image covering a Z range of 554 µm. Image rendered with Imaris (Image credits Marco Campinho, Universidade do Algarve and Claudia Florindo, Andor Technology)
Furthermore, imaging of thick live samples is also possible. BC43 can readily be integrated with an incubation chamber to maintain the correct incubation conditions (i.e. temperature, humidity and CO2). For example, the BC43 has been used to study live Zebrafish samples; the experiment aimed to address angiogenesis in the developing zebrafish embryo. Embryos were successfully imaged for 48h without signs of any phototoxicity or photobleaching, highlighting that Andor Benchtop Confocal - BC43 is highly capable for imaging live, deep thick tissues.
Last, in order but not in importance, BC43 can also deliver high resolution confocal images as fast as 15 fps in full-frame mode and at 44 fps when imaging with a 512x512 window.
Movie 1 - Live Imaging of Zebrafish Vascular Development. Live image of transgenic zebrafish hindbrain vascular development (in Cyan) combined with DPC (differential phase contrast) in grey. Imaging was performed for 48h. Image was acquired with Andor Benchtop Confocal – BC43 time lapse option combining DPC (differential phase contrast – grey) and Confocal imaging (Cyan) using multiple tile acquisition and montage. 20 tiles were acquired to compose the image each tile had 175 slices, over a Z range of more than 600 µm. (Image by Marco Campinho, CBMR Universidade do Algarve and Claudia Florindo, Andor Technology)
In summary, the confocal imaging modality of BC43 can be used to acquire live or fixed samples, and when appropriate, in combination with transmitted light microscopy. In addition, users can image up to four different fluorochromes using the confocal option.
As discussed in the article Overview of Microscopy Techniques for Life Sciences, confocal microscopy might not always be the best imaging option for fluorescently stained samples.
For live-cell imaging, and especially for capturing fast, dynamic events in thin and photosensitive samples, widefield can be a more suitable imaging modality than confocal. Taking the above into consideration, Andor has incorporated widefield fluorescence imaging modality into the new benchtop confocal - BC43.
Furthermore, by taking advantage of Clear-ViewTM Deconvolution that is integrated into Fusion software, the resolution of widefield images can be increased markedly. By using Clear-ViewTM, the characteristic blur of widefield images will be removed. Consequently, it will increase the signal to noise ratio of the image and its resolution. In fact, it should be noted that an adequately sampled widefield image processed with Deconvolution can have a resolution comparable to a confocal image.
The widefield imaging modality is well suited to long duration live cell studies. In one study the BC43 was used to acquire images every 8 min for 24 hours. (Cells were in a controlled temperature environment but without humidity or CO2 control.) The results showed that after 24h of imaging, photobleaching could not be detected. After 24 hours of imaging, many cells started a second round of cell division. This observation indicates that the sample did not suffer from phototoxicity.
With an incubator chamber controlling CO2 and humidity, it is possible to image for several days in widefield or confocal.
Movie 2 - BC43 Imaged Mammalian Cells in CO2 Independent Media for 24h. This video shows a merged image of DPC and Yellow widefield channels. It can be observed that even at the end of the movie more cells were starting cell division indicating that even after 24h, and without CO2 in the incubator chamber, the cells did not show any sign of phototoxicity. Indeed, no photobleaching can be seen in this movie. Hela Cherry tubulin were imaged at 37ºC, images taken every 8 min for 24h. Fusion protocol had the ClearView™ deconvolution option activated. The multi-position option was activated, and 10 imaging positions were acquired in this protocol. The Video shows one of the 10 different imaging positions acquired. For each position DPC and the Yellow widefield channel were acquired. Twelve stacks covering a range of 16,5 µm were acquired for each channel. The presented video was processed with Imaris software and is a maximum intensity projection of the acquired stacks. Image credits Ines Baião-Santos, Álvaro Tavares – Universidade do Algarve, Claudia Florindo – Andor Technology
For transmitted light microscopy, the user can select two imaging modes with the BC43, these are Brightfield, and an Andor patent pending technique called Differential Phase Contrast (DPC)
Brightfield is the most straightforward and widely used technique in transmitted light microscopy, the optics and alignment are simple. In Brightfield, light passes through the less dense areas and is absorbed in denser areas of the sample, generating contrast. This technique is, therefore, good for samples with different degrees of density. However, for thinner samples, and samples that do not have large enough density gradients (e.g., monolayer cells), the visibility of the cells and even structures inside, such as the nucleus, becomes difficult since the sample will be largely transparent. In this case, Andor’s patent pending Differential Phase Contrast (DPC) will be the ideal choice (Figure 4).
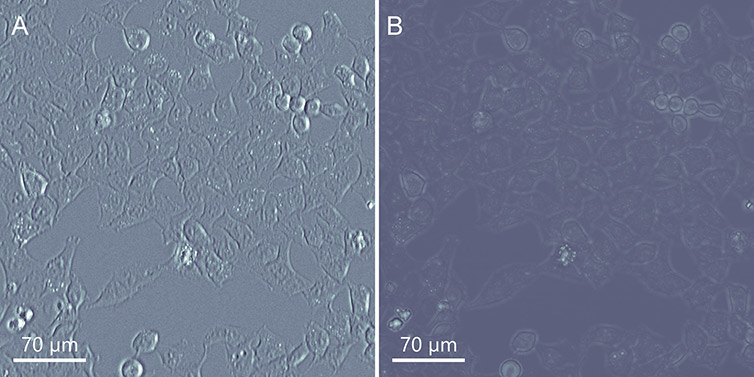
Figure 4 - Comparing the BC43 Transmitted Light Imaging Modalities in Flat Mammalian Cells. DPC (Differential Phase Contrast) on the left and Brightfield on the Right. A) DPC image B) Brightfield image. As it can be observed the DPC image presents much more useful information on the cells than the brightfield image (which is nearly transparent in this type of sample).
For thicker samples, DPC can offer an advantage to an image, enhancing the image with a hint of 3D structure, making the features stand out even more. Nevertheless, the reader should keep in mind that Brightfield could still be a good imaging option in some cases (Figure 5).
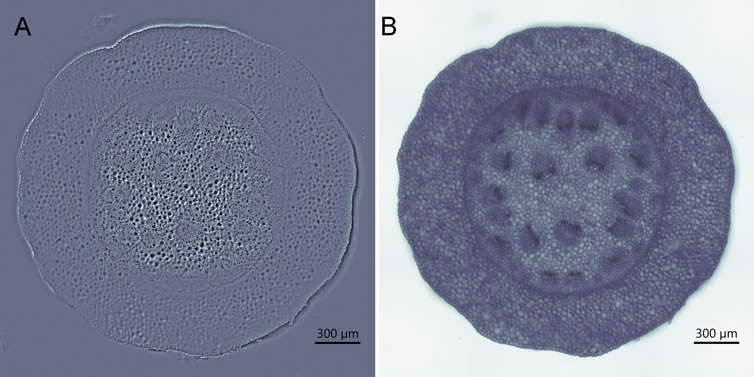
Figure 5 - Comparing the BC43 Transmitted Light Imaging Modalities in Convalaria Tissue. DPC (Differential Phase Contrast) on the left and Brightfield on the Right. A) DPC image B) Brightfield image. As it can be observed either the DPC or Brightfield imaging modalities are a good option to use as transmitted light, as is shown here for convallaria. User should select depending on the application.
In summary DPC does not need: specialised objectives (like phase contrast objectives), polarisation optics (as in DIC), and it can be used to image birefringent specimens.
Furthermore, DPC allows consistent performance across the image area, all the time it is not dependent on adjusting angles or maintaining polarisation.
Very importantly, unlike DIC, DPC images can be obtained through plastic dishes, which is practical, if not essential for numerous biological imaging applications.
For BC43, the transmitted light techniques are accessible by a few clicks of the mouse since there is no need for user alignment in either Brightfield or DPC. With BC43 the user does not need to set up Kohler illumination or align phase-plate/phase ring alignment.
For a better understanding of transmitted light microscopy techniques, please go to the article Overview of Microscopy Techniques for Life Sciences.
One of the difficulties with microscopy systems, is how to combine the high technical performance and a wide range of features required by today’s researchers in a way that is truly easy to use. Do imaging experiments really need to be so complex to set up and require many hours of training? The BC43 was designed with this challenge in mind: to make it accessible and easy for every user, but still deliver all the features needed from routine acquisitions, through to complex multi-dimensional experiments.
With the BC43 we carefully studied how real researchers interacted with the hardware, samples and software. This resulted in an instrument that users became comfortable and competent using very quickly. We could say that a new user of BC43 goes from 0-100 understanding in as little as 1 hour.
If labs/imaging facilities train the users on the system, a quick overview of the system in 30 minutes to 1 hour will be more than enough to get users going independently. BC43 will therefore free up facility and lab managers to complete the more demanding tasks while delivering 3D confocal microscopy images. The graph in figure 6 compares the rate of learning to become competent using BC43 and point scanners, with and without a tutor.
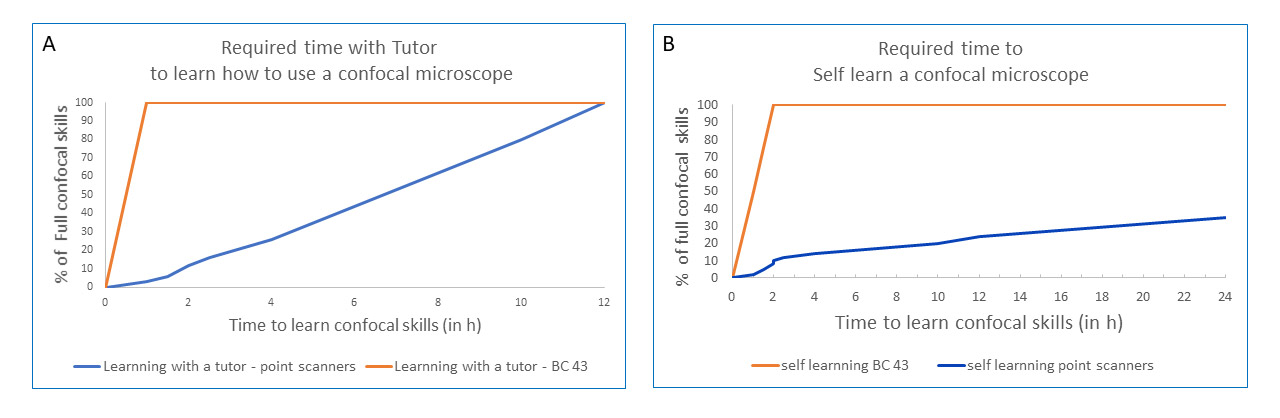
Figure 6 - BC43 Delivers a Super-Fast Learning Curve. Graphs show a comparison between the learning time needed to become competent using the master BC43 (blue line) and a point scanner (orange line). It can be observed that the time need to master the equipment is much shorter when using BC43. A) Time need to master the confocal microscope when a tutor is teaching how to use the microscope. B) Time need to master the confocal microscope when the user teach himself by accessing training materials.
Alessio Carletti, PhD student from the Faculty of Medicine and Biomedical sciences, University of Algarve, compares the learning on the BC43 with his previous experience on point scanner confocal:
"Compared to point scanners, I felt that the learning curve on BC43 was much faster, and the path of getting to operating the scope was smoother.
With a point scanner, there is a lot to take care of manually. Starting from the sequence of tasks to get the machine up and running: turn keys, press buttons, turn the fan on, then you must set up the best light-path for each channel, take time to select the filters etc... Overall, the whole process on a point scanner is quite laborious. It took me 3-4 days of assisted training to fully master it and feel sort of handy."
With Andor BC43, the process is fully automated. You literally only have to press the on/off button. I think it is a very nice instrument."
BC43´s fast learning curve is delivered by the easy workflows available to users that allow the acquisition of a simple 3D image or even complex acquisitions like Z stack, montage, etc., with minimal software interaction.
Immediately after loading the sample, with 2 to 3 clicks, users can set up complex experiments such as 3D-Z stack acquisition, multi-position acquisition, and montage.
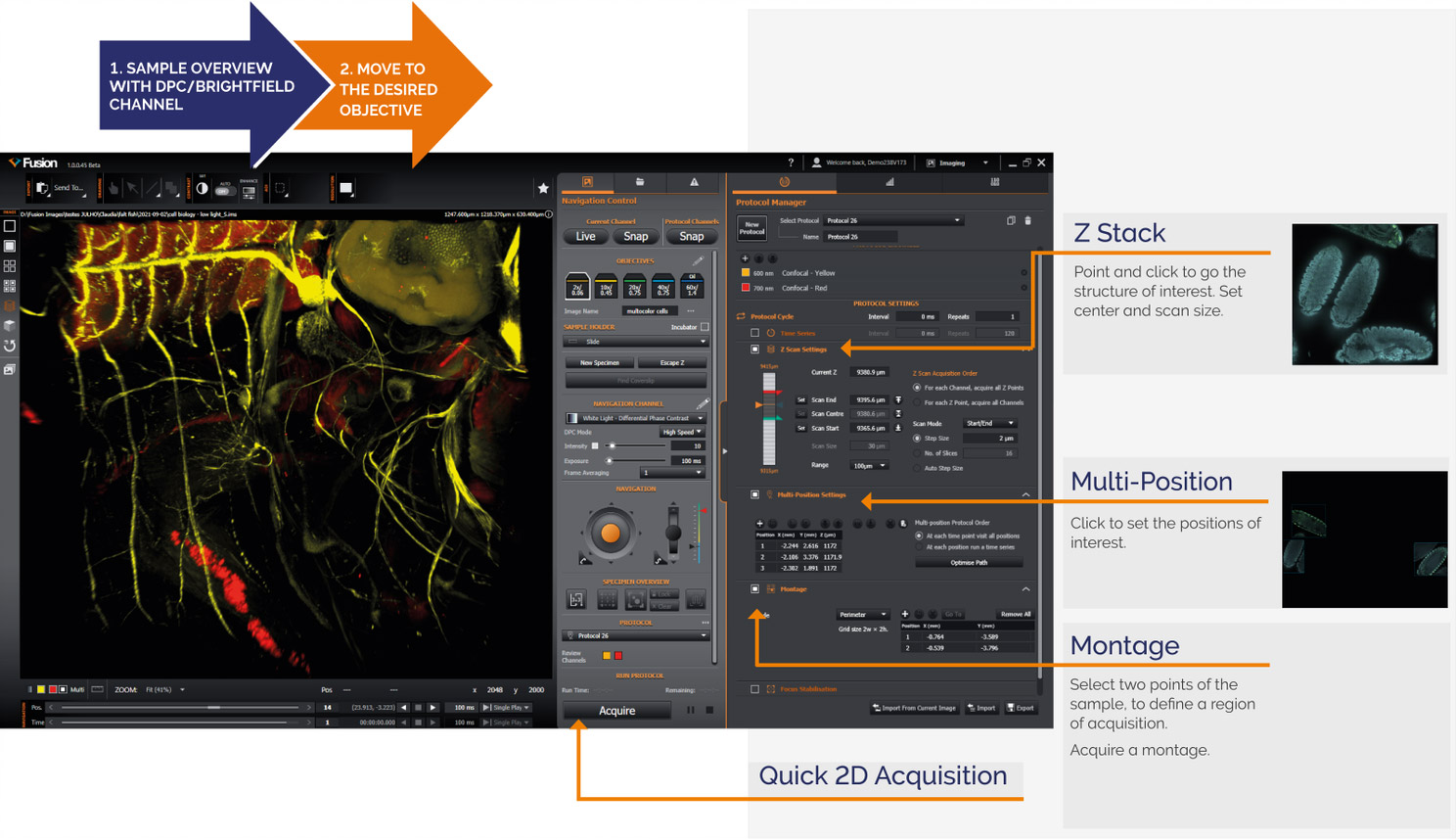
Figure 7 - Overview of Fusion BC43 Interface and Workflows
Further, all workflows can be easily combined, allowing the user to acquire multi-dimensional experiments with the touch of a button.
Some detailed examples of BC43 fast workflows are as follow:
a) Two-step Z stack acquisition:
-20211103152821.png)
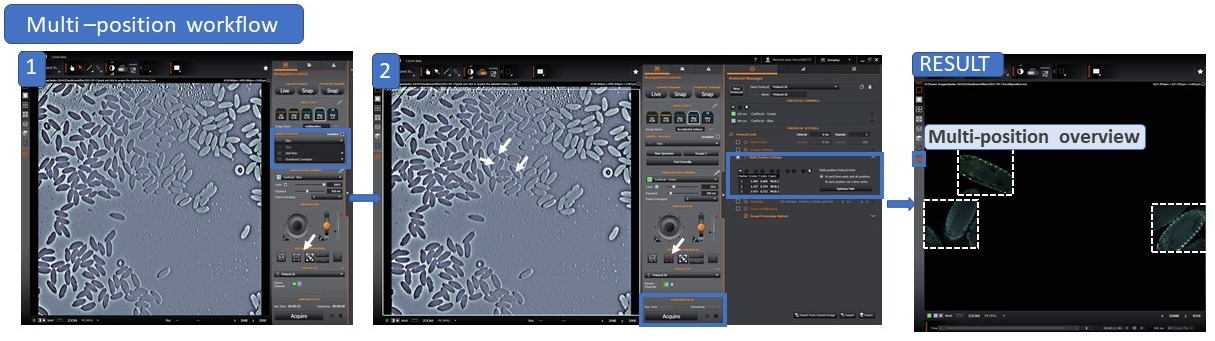
b) Two-step Multi-position workflow:
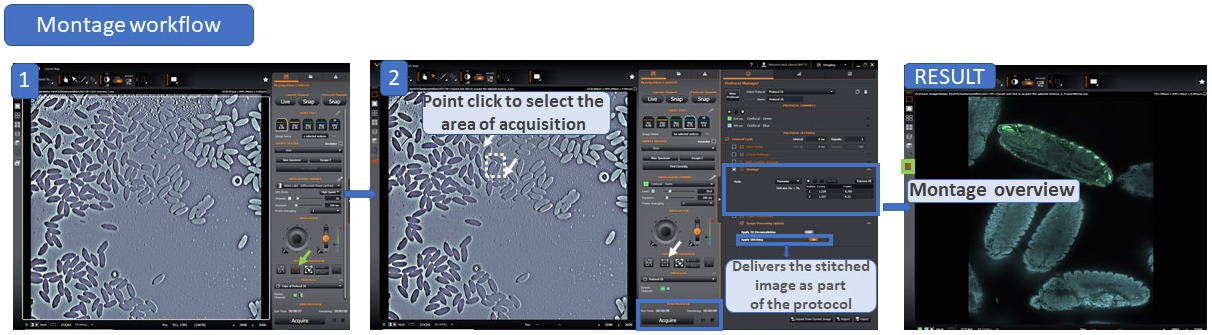
c) Two-step Montage Workflow:
In summary, the simplicity of workflows and the flexibility in combining multiple dimensional experiments deliver a complete multimodal system that meets the needs of users with any level of microscopy experience, from the undergrad student just starting out, to the post-doc who sets the direction for a lab.
It can be difficult for new users, to find a sample and bring it into focus. Andor’s patented Focus seek & lock mechanism was designed and implemented for the first time in BC43 to overcome such hurdles.
Focus seek & lock (Finding the Sample)
The 2x objective in BC43 has a very large depth of focus, and as such, it delivers almost instantaneous focus of any sample. In addition, the workflow overview (described above) will allow users to center the structure to be imaged.
Image acquisition can then proceed by moving to the next air objective and automatically finding the sample using the Focus seek & lock mechanism of BC43. With this simple workflow, the sample is brought into focus instantly.
Focus seek & lock (Keeps the sample in Focus)
Andor Benchtop Confocal - BC43 is designed for 3D imaging allowing multi-dimensional experiments; it can accommodate the acquisition of multiple tiles, positions and combine them with time-lapse imaging. Not surprisingly, during a large sample acquisition (capturing multiple tiles) and when imaging live samples (time-lapse imaging), there is the need to ensure that the sample stays in focus during the whole experiment.
Once the sample is brought into focus, the Focus seek & lock mechanism can be activated in the protocol, and it will automatically "lock" the focal position. The software verifies the focal plane at small time intervals, ensuring that the sample is kept in focus, for hours or even days, depending on the experimental design.
Movie 3 - Multi-position Imaging of Mammalian Cells with BC43. Mammalian cells were imaged with BC43 using confocal imaging mode for over 4h. At each time point, 4 independent positions were imaged and for each position we acquired 3 channels and 15 Z stacks. The movie shows the 4 positions playing simultaneously. As it can be observed the focus Seek & lock mechanism was able to keep the focus of the sample while navigation in the different positions. Image credits Ines Baião-Santos, Álvaro Tavares – Universidade do algarve, Claudia Florindo – Andor Technology.
Productivity is vital for success and can be achieved in several ways: by being more organised, pre-scheduling and automating repetitive tasks, etc. For example, in microscopy, automating the acquisition of independent positions increases productivity. and frees the researcher to undertake other tasks while the acquisition is running.
Andor Benchtop Confocal - BC43 uses an effortless workflow and simultaneously allows the acquisition of complex multi-dimensional experiments without adding to the complexity. Furthermore, acquisition of multiple positions can be combined with any other acquisition options: either multiple channel (acquiring independent fluorochromes), Z stack, timelapse and even montage.
The multi-position option is also incorporated in the multi-well feature allowing the user to acquire multiple positions on tissue culture well plates from 6 to 96 wells. Importantly, unlike DIC, DPC delivers high contrast images through plastic. As such, when the focal distance of the objective allows (10x will most often work well even for thicker well plates), the cells can be imaged on their growing tissue culture environment.
In Figure 8, an example of this functionality, showing presenting a three-position acquisition, each with nine tiles and combined with a Z stack at each position.
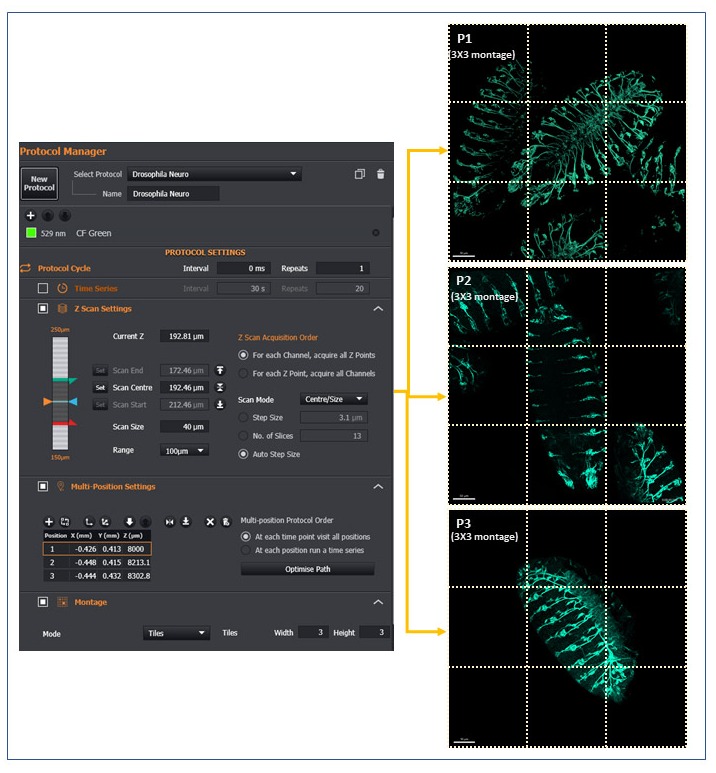
Figure 8 - Example of BC43 Multi-position Montage Acquisition. Drosophila embryos stained with neuronal marker were set up on a multi-position/montage acquisition. Each image is a montage of a 3X3 field at the selected position, 101 z slices covering 50 µm range. Each of the nine tiles acquired is highlighted with dotted lines. (Image by Álvaro Tavares – Universidade do Algarve & Claudia Florindo – Andor Technology)
BC43 offers imaging processing options at the distance of one click on the protocol. The processing of the images can be activated in the protocol and will start soon as acquisition is completed. By selecting these options, BC43 delivers, upon protocol completion, both the unprocessed and the processed image.
Processing options on the protocol tab of BC43 include:
As discussed in the article "Overview of Microscopy Techniques for Life Sciences", Deconvolution is the process by which the distortions that arise in a microscopic image are mathematically corrected. Such distortions, named convolution, are unavoidable and intrinsic to the use of the various optical elements of a microscope.
BC43 acquisition software integrates Andor's high-quality GPU-based deconvolution algorithm ClearView-GPUTM. It is important to realise that the deconvolution algorithms work iteratively, iteration by iteration increasing the image quality, and demanding high processing power. Therefore, if the Deconvolution is CPU-based, image processing can be highly time-consuming. Furthermore, when users acquire large scale 3D data sets, time-lapse, or a combination of multi-dimensional possibilities, the extra time on processing images gains additional importance.
Andor's ClearView-GPU Deconvolution delivers results up to 50x faster than CPU-based methods and up to 10x faster than other leading GPU-accelerated packages, especially for larger datasets, even when iteration acceleration is disabled.
Further, in BC43, the Deconvolution can be activated in the protocol, Therefore, the user will have the raw data and the deconvolved image soon after the acquisition is finished.
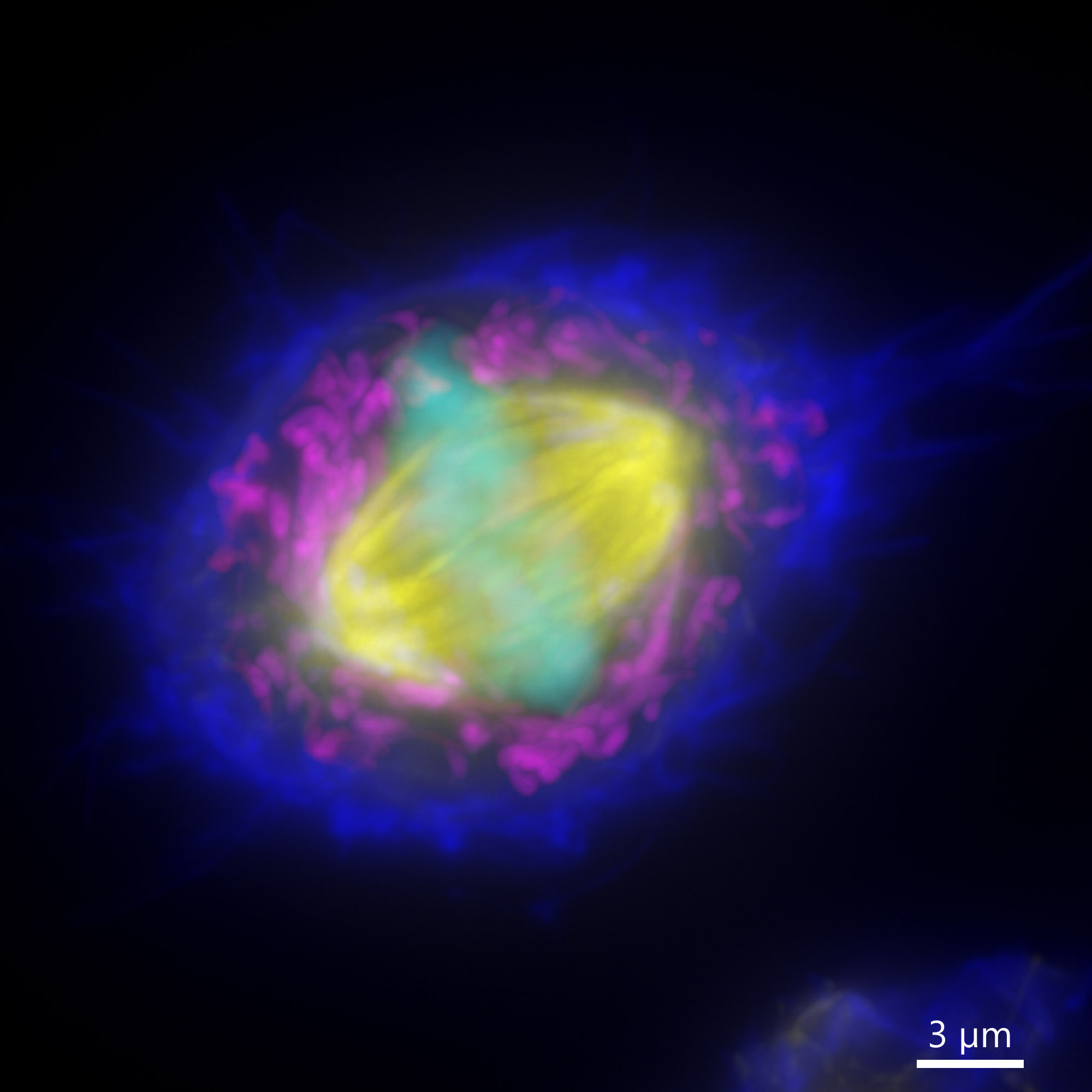
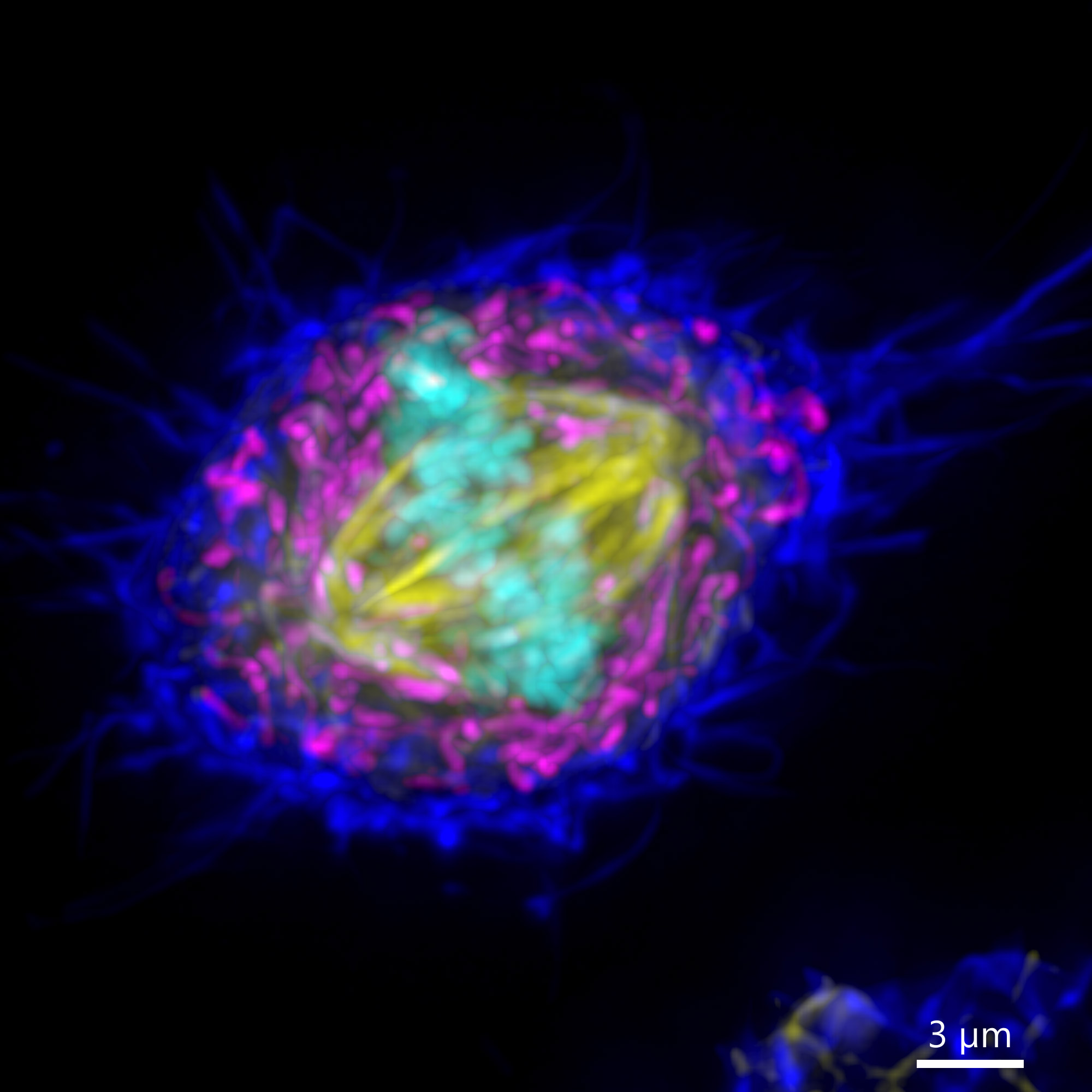
Figure 9 - Widefield BC43 Image of a Mammalian Cell (Before and After Deconvolution). Mammalian cells were imaged using BC43 using the widefield acquisition mode. Image is a maximum intensity projection of 70 Z stacks covering a range of 10 µm. Raw data (left) and deconvolved image (right) were delivered, by selecting the deconvolution option on the protocol. Actin is shown in dark blue, mitochondria in yellow, microtubules in magenta and the DNA in Cyan. (Image by Claudia Florindo – Andor Technology)
As expected, when using higher magnifications in a microscope, the field of view will be reduced. However, microscopists often want to acquire images at higher magnifications while also needing to acquire the whole organism or image large areas of a sample. Such tasks may turn out to be incompatible for most samples since the higher the magnification used in the acquisition, the smaller the acquisition field of view.
One way to overcome this problem is to acquire multiple fields to cover a larger imaging area, then combining all tiles into a single image. This process can be done manually or automatically, and the complexity of the process increases when the image to be stitched is a complex data set (multi-channel 3D or 3D+time). In addition, other complexities such as the overlap between the images needs to be enough to allow proper stitching.
Finally, the microscope must provide a flat, uniform illumination across the field of view. When the illumination is not uniform, the edges of the individual tiles used in acquisition can be clearly visible in the final stitched image and the image appears like a mosaic of tiles and not a uniform complete image
BC43 has incorporated Andor's patented Borealis Uniform illumination. This highly flat illumination, combined with the stitching algorithm integrated into Fusion Software, delivers a seamless stitched image like the one seen in movie 4. In addition, stitching can be activated directly as part of the protocol, delivering a stitched image soon after the acquisition is finished
In BC43, both Deconvolution and stitching can be activated in the same protocol. In such cases the stitching will follow after Deconvolution. Again, following the system's simplicity, activating both takes just a single click on the protocol.
Movie 4 - Seamless Zebrafish Intestine Stitched Image Acquired with BC43. Image was acquired using the confocal imaging modality of BC43, with 4 imaging channels, 77 stacks and 28 tiles. The full stitched image is composed of a total of 8624 images. The deconvolution and stitching options were both activated on the protocol. Image shows the Zebrafish intestine. Sample courtesy of Julien Resseguier, at NorMic - University of Oslo.
BC43 is a high-quality benchtop confocal system. It contains the capability that you would expect from a full-size confocal imaging platform but compressed into a small 0.5x0.6x0.4m footprint without compromising image quality or acquisition speed. Since BC43 has such a small footprint, it can easily fit on a bench as part of a laboratory workflow. In BC43 the sample and imaging is contained in a enclosed space, eliminating the need for a darkroom. With the system on the bench, researchers can monitor how the imaging is running while preparing their next sample or working on other experiments. (Figure 10).

Figure 10 - View of BC43 in the Lab. The image clearly shows the small footprint of Andor Benchtop confocal. Due to the integrated anti-vibration mechanism, BC43 can be placed on the bench side by the side of centrifuges, orbital shakers, etc.
BC43 has an integrated anti-vibration mechanism that allows the system to be used on the bench while researchers perform experiments or even use other laboratory equipment.
Users also benefit from an ergonomic and user-friendly joystick that facilitates sample loading and alignment with the imaging objective, and allows them to focus and move the sample while imaging is being set up.
"It fits on a bench and because it's a box we don't need a dark room, and with integrated suspension, we can go on working while imaging is taking place. In addition, the vibrations are not seen on the image; as such, it will not impact quality." - Álvaro Tavares, Professor and Group leader, CBMR – Universidade do Algarve
A 2x objective is integrated into the standard BC43 package. Using this objective, microscopists can quickly browse through the sample, acquire an overview of a large area, select the area to be imaged and proceed to the imaging objective to acquire the data. (See Figure 7 for workflow examples). The use of the 2x objective combined with the joystick also facilitates easy sample navigation and alignment. For a customised user experience, it is possible to adjust navigation and focus speeds on the joystick.
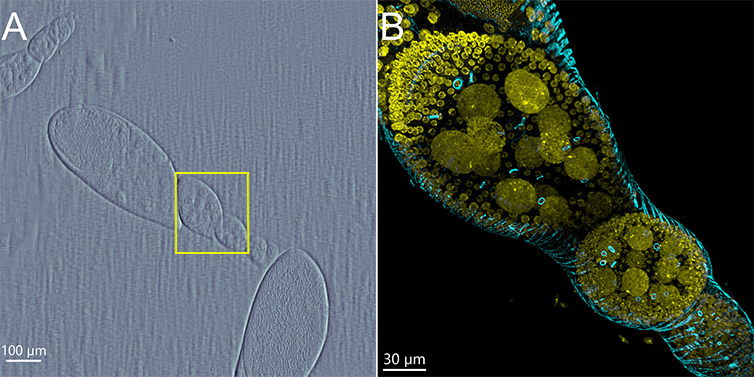
Figure 11 - Drosophila Egg Chamber Imaged with BC43. A) Overview image, using the DPC imaging channel and 2x objective. The area in yellow was selected to be imaged with the 60x objective. B) Image of Drosophila egg chamber upon selecting the area with the previsualization with the 2x objective. The image is a maximum intensity projection of 309 Z planes acquired covering a range of 93 µm The image was further deconvolved and rendered in imaris. (Yellow-DNA, Cyan-actin). Image credits Rui Silva – Universidade do Algarve, Claudia Florindo – Andor Technology.
BC43 has an integrated multi-pinhole disk to allow fast confocal imaging with any objective (up to speeds of 44 fps). The optimised spacing between the pinholes delivers high resolution when using high magnification objectives (60X and 100X); but also allows imaging deep into the samples when using lower magnification and /or long working distance objectives. (Data acquired with the system demonstrated the ability to image more than 500 µm in cleared flatfish samples. Fig. 3)
Additionally, the disk can automatically be removed from the imaging path, allowing widefield imaging. This imaging modality can be beneficial for thin or very sensitive samples that do not benefit much from confocal, but benefit from the lowest possible illumination intensities.
Finally, it also delivers transmitted light options with Brightfield and DPC (discussed above and in the article "Overview of Microscopy Techniques for Life Sciences”.
Confocal and Widefield imaging have four different laser lines available. Laser lines supplied with the system are 405, 488, 561 and 638 nm.
The transmitted light modalities are implemented using a condenser-free high uniformity Broadband white light LED light source.
In summary, the above features allow the BC43 to be pre-configured with a total of 10 imaging channels:
The laser wavelengths cover the most common fluorochromes used in life sciences allowing the user to image even with different modalities in the same protocol to meet the needs of each individual experiment.
The system is designed to deliver fast imaging both in confocal and widefield mode. Users can set a workable minimum exposure time of 10 ms and still get acquisition speeds as fast as 15 frames per second when using the full 4 Megapixel chip and up to a maximum of 44 frames per second at 512X512 resolution.
The reader should note that the “real-world” frame rates are defined, considering an exposure time that could deliver meaningful data (signal collected in 10 ms) and not an exposure time of e.g. 0 ms (that would technically be the fastest imaging possible). Exposure times of 0 ms will of course not deliver an image, just black images absent of any meaningful signal. Therefore, we have provided frame rates that relate to real, imaging experiments.
| Sensor Size | Maximum Speed (10 ms Exposure) |
| 2048 x 2000 | 15 FPS |
| 2048 x 1024 | 19 FPS |
| 1024 x 1024 | 30 FPS |
| 512 x 512 | 44 FPS |
Table 2 - Acquisition Speeds of BC43
When comparing the frame rates of microscopes, it is important to consider the resolution and field of view at which those frame rates are delivered.
An image acquired at a faster frame rate, achieved with a dramatically diminished field of view or the highly binned camera chip will probably look impressively fast on paper, but in reality, offer unusable data. As such, we advise users to ensure that the system is fit for purpose and, when needed, test the system's speed with typical settings for your samples. The image delivered at the desired frame rates should also deliver high quality images with valuable and meaningful data.
Movie 5 - EB1 - GFP Fast Imaging Using BC43. Hela cells expressing EB1-GFP were imaged, in confocal mode, for 6 min at a frame rate of 2 frames per second. It can clearly be observed the microtubules plus end tips growing from the centrosomes of the cell that is going into mitosis. Image credits Ines Baião-Santos, Álvaro Tavares – Universidade do Algarve, Claudia Florindo – Andor Technology.
In conclusion, the frame rates and sensitivity of BC43 allow it to deliver meaningful results in fast imaging applications. Examples of such are microtubule tracking (EB1 /Eb3 plus-end microtubule tips) and even calcium waves.
The stage integrated within the BC43 is fully motorised in XY and Z.
Another key feature is Focus Seek & Lock that combines with the precision and resolution in XY and Z to ensure focus stability is maintained even on large multi-field image acquisitions. Furthermore, this focus stability is also achieved even when multi-field / multi-position acquisitions are combined with extended time-lapse imaging.
Sensitivity and resolution are critical parameters in fluorescence microscopy. Andor Benchtop Confocal - BC43 utilises a high sensitivity 4 Megapixel sCMOS detector with 82% peak quantum efficiency (QE), ultra-low noise and 6.5 µm pixel size. The high QE and low noise floor ensure the detection of faint signals at any magnification, and 6.5 µm pixel size allows maximum resolution for detailed, vibrant images.
The large diagonal of the sensor (18.4 mm) delivers a field of view that is as large as 1.84 mm when using the 10x objective. Significantly, this combination of a large field of view and small pixel size will dramatically increase productivity since much more information will fit in a single image. For example, a whole zebrafish with a 1.84 mm size can be imaged in one single field (using the 10X objective).
Finally, the 16-bit dynamic range of the sensor delivers images that can have up to 65,535 grey levels, which enables the bright and faint signals to be captured in a single image. This extended bit depth will also be extremely important when doing image analysis and quantification since it permits more refined distinction in signal intensities and, as such, better image quantification.
As explained above, BC43 allows extensive montage imaging by acquiring multiple tiles that can be further combined to compose an image of the entire sample. Furthermore, both montage and stitching are integrated into BC43. Thus, the user can capture multiple fields of a sample and then combine them.
That alone would deliver the full sample, but still, the image would not be of great quality as many researchers find when they combine image tiles using other imaging systems. Therefore, Andor has developed and patented Borealis Perfect illumination. Borealis allows flat illumination of the full field to be imaged with minimal roll-off at the edges. The benefits of this are most easily observed in image montages with exceptional uniformity maintained across the stitched image.
Borealis combines high-frequency laser homogenisation with an optical fiber specially selected to match the microlens disk. As a result, BC43 delivers high throughput, low background, and high spatial uniformity. Furthermore, Borealis uses a multimode fiber leading to optical power stability over the long term, which will consequently reduce maintenance and its costs as well as increase productivity.
The overall result of Borealis – perfect illumination is:
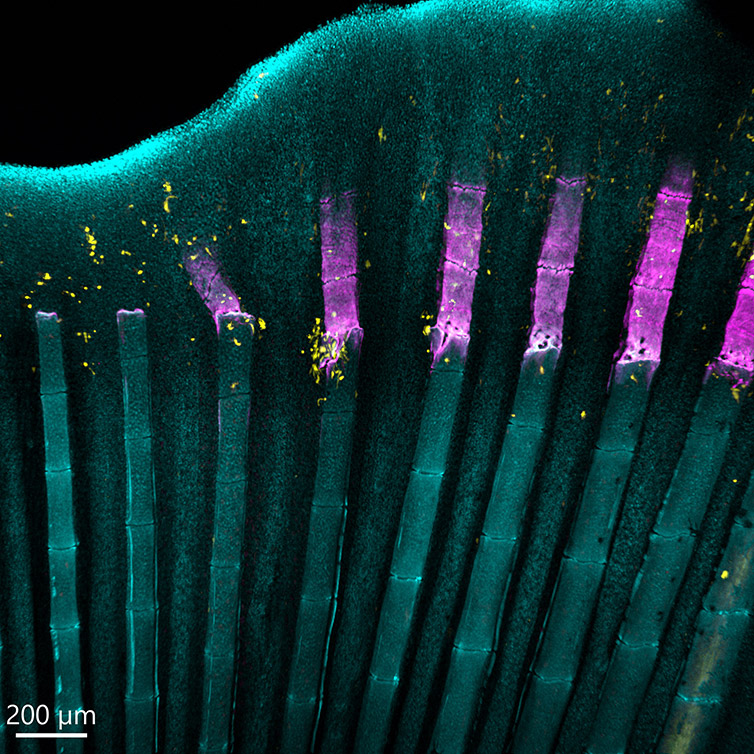
Figure 12 - Zebrafish Fin Imaged with BC43. Image of a zebrafish fin showing the process of bone regeneration. Image shows the perfect stitching of 4 imaging fields. We acquired three channels and 51 stacks for each field, covering a Z range of 174 µm. This image shows the adult zebrafish (Danio rerio) regenerating caudal fin at four days post-amputation. It can be observed the newly formed bony tissue in purple (calcein staining) and cathepsin k+ cells (the osteoclasts) in yellow, DNA is in Cyan. Image credits Alessio Carletti – Universidade do Algarve.
In summary, we have explained in detail the imaging modalities, hardware and software features of the BC43.
Andor Benchtop Confocal - BC43 delivers:
These features make BC43 a wonderful system for multiple life sciences applications.
We believe that after testing BC43, users will be convinced that:
BC43 is THE choice for the Benchtop Microscope Imaging System
In the final section of this article, we will conclude by briefly discussing some key life sciences application of BC43.
The imaging applications of BC43 are intrinsically related to the techniques and technologies that this equipment allows. As it can be observed in table 3, there are several imaging techniques possible with BC43 allowing for use in many different applications:
| Technique | Technology | BC43 |
| Transmitted Light Microscopy | Differential Phase Contrast (DPC) | ✔ |
| Brightfield | ✔ | |
| Widefield Imaging | Laser Widefield | ✔ |
| Up to 4 Channels | ✔ | |
| Confocal Imaging | Up to 4 Channels | ✔ |
| Imaging Modes (and combinations of) | Confocal | ✔ |
| 2D Imaging | ✔ | |
| 3D Imaging | ✔ | |
| 3D Tile Imaging | ✔ | |
| Deconvolution | ✔ | |
| Multi-well | ✔ | |
| Stitching | ✔ | |
| 3D Stitching | ✔ | |
| Multi-position | ✔ | |
| Time lapse Imaging | ✔ |
Table 3 - Imaging Technology and Methods of BC43
Considering the wide range of imaging possibilities of the BC43, we have presented a small selection of examples that the BC43 will prove especially well suited:
The example model organisms can be imaged live or fixed depending on the biological question. It is possible to use up to four different fluorochromes in a single sample. Furthermore, when appropriate, transmitted light microscopy imaging techniques can easily be combined with confocal or widefield.
Some example imaging applications are:
In conclusion, the system has been thoroughly tested to understand the applications in today’s busy laboratories. As has been outlined in this document you can see that the BC43 is exceptionally versatile and capable of delivering outstanding results in a multitude of different applications and wide range of model organisms in ways that are not possible in other imaging systems.
In summary, we can conclude that:
BC43 is a revolutionary benchtop 3D confocal imaging that will quickly turn into a workhorse for your lab or imaging facility.
Date: November 2021
Author: Dr Claudia Florindo
Category: Technical Article
