Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Immunofluorescent labeling has established as a mainstay method for specifically labeling biological macromolecules, by virtue of the exquisite specificity offered by antibody binding sites for their corresponding antigens. Fixed cells and tissues are very common specimens for immunological labelling. In such specimens, fluorescently labeled antibody conjugates are an important tool for determining both the presence and the sub-cellular localization of an antigen, and in some cases can also be quantified to yield relative concentrations of sub-cellular biomolecules.
The most common protocols for Immunofluorescence labeling are Direct and Indirect. Direct labeling involves incubating the sample with a fluorophores-conjugated antibody that is specific to the antigen of interest. With Indirect labeling, the specimen is incubated first of all with an unconjugated antibody, specific for the antigen under study. Then a second fluorophore-conjugated antibody is introduced that is specific for the first antibody.
Immuno-labeling has proven highly effectual in helping determine the localization and function of sub-cellular proteins. One method is to first bring about expression in the cell of an epitope-tagged protein, without affecting cell physiology, making use of an appropriate expression vector. A raised antibody, specific to this epitope, can then be used to specifically label the expressed protein of interest, either by direct or indirect means.
Immuno-labeled specimens are examined under a fluorescent microscope, often by widefield epifluorescence microscopy. Widefield epifluorescence microscopy has developed into a universally accessible technique for study of fluorescently labeled cells and tissues. Over the last few decades, this drive has been accelerated by improvements in fluorescent probes, labeling chemistry, optical instrumentation (such as filters and objectives) and detector technology.
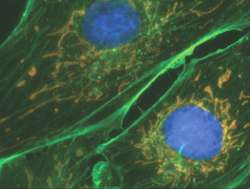
Color merged image from multi-labeled immunofluorescence fixed cell sample. Recorded with the iXon3 865 front-illuminated on a widefield epifluorescence microscopy set-up.
The widefield technique involves flood-illumination of the field of view by a wavelength or small wavelength range (often though use of an excitation filter and arc lamp). The stoke-shifted fluorescent emission transmits though the dichroic, that was initially used to reflect the shorter wavelength excitation light onto the sample, gets optically filtered once again by an emission filter (often called barrier filter) and is then focused onto the detector of the scientific camera.
Often, specimens are labeled with multiple fluorescence labels, some by immuno-specific means, some not (e.g. the DNA-intercalating dye DAPI). The idea is then to record a series of images, each corresponding to a fluorescent label. One must therefore make use of a series of epi-fluorescent filter blocks (excitation, dichroic and emission filters) that are specific to the dyes of interest. It is also possible to use mutli-band dichroic filters that do not have to be swapped out for each image.
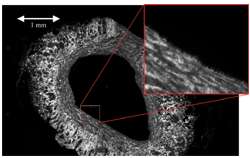
Montage scan of an immunofluorescence labeled blood vessel section, created from 108 (12x9) fields using Andor-MF module for montage scanning and stitching. Data was acquired using an iXon3 885 camera and Ludl Biopoint stage on an Olympus BX51 with 20x/0.5 NA objective. Courtesy of Dr Stephen Goodall, Leicester University, UK.
The image quality of widefield epifluorescence microscopy on immunofluorescent samples can be compromised in a number of ways:
1. For each dye probed, the ideal situation is to achieve an image that is untarnished by the emission of the other dyes. This is rarely achieved in practice, due to the spectrally broad nature of both excitation and emission bands, resulting in spectral overlap between dyes, i.e. filtered excitation light for one dye will excite a little bit of a spectrally neighboring dye, and a filter to collect emission light of a specific dye will also collect a small percentage of light from other dyes. The solution to achieving highest contrast, is to use specific sets of narrow-band excitation or emission filters, positioned to have minimal spectral overlap with the other labels. This can compromise signal to noise however, since the narrow-bandpass nature of such filters means that only a fraction of the available excitation or emission light is being transmitted to the sample or CCD respectively.
2. A common issue with Immunofluorescent labelling, is non-specific binding (also known as background staining) of antibodies to regions of the cell that they are not specific to via their antigen-binding site, instead attaching via other types of molecular interaction. This can result in reduced contrast and can be particularly problematic when trying to find weakly expressed proteins for example. Fortunately the problem is addressable and there are a number of well-documented precautions that can be taken during sample preparation to ensure that non-specific binding is minimized. One very effective step towards minimizing this form of background, is to employ direct labelling protocols. Whilst indirectlabeling can boost overall signal intensity, the use of secondary antibodies results in extensive non-specific binding, compromising signal to background ratio. Therefore it is more beneficial to make use of a sensitive detector combined with direct protocols.
3. A common concern is fading of the signal. Often to achieve an adequate signal to noise, a high intensity excitation light must be employed, that also causes accelerated photobleaching of fluorophore labels. "Anti-fade" mounting media agents can be used that retard the rate of photo-bleaching, but the most effective ones invariably cause emission intensity to deplete also, and can sometimes result in higher photon background and diffused fluorescence.
4. Another negative effect of widefield is "out of focus fluorescence". This is due to light from outside of the focal plane making it through to the detector. One way to avoid this completely is not to use a widefield technique, but instead make use of a highly optimized epi-fluorescent confocal set-up, such as the Andor Revolution (see Live Cell Confocal Microscopy in the Biology Application Range for more detail).With widefield though, it is possible to apply deconvolution algorithms to collected images to minimize the effects of out of focus haze and improve contrast. For such algorithms to be successful, signal to noise and resolution need to be optimal. Andor iQ software offers a highly effective deconvolution module called ClearView.
Explore our related product range below...
