Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Challenge Background
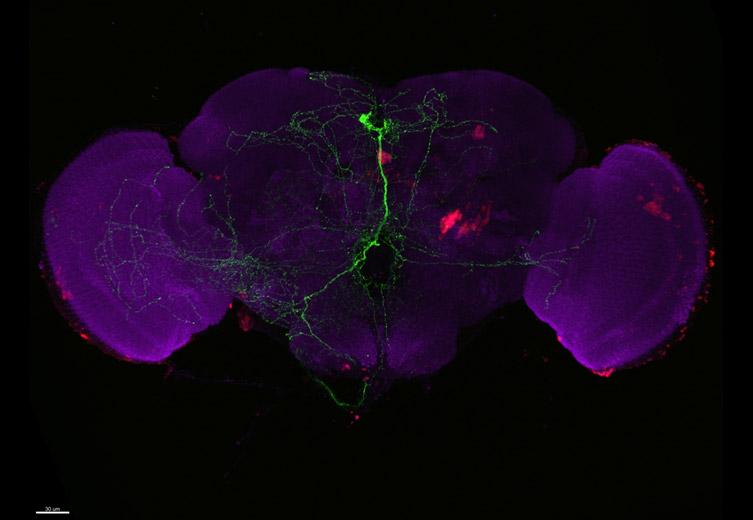
Research projects transition from findings made at the single cell or subcellular level to more complex multicellular invertebrate and vertebrate model organisms to determine clinical relevance. Model organisms include Zebrafish, Drosophila, Wasp, C.elegans and sea urchin. For these thicker specimens, to see detail at depth and avoid out-focus-signal, laser scanning confocal microscopy is traditionally used. However, there are several challenges with laser scanning confocal microscopy (LSCM), which scans a point of laser light across a sample building an image pixel by pixel and line by line. Three key challenges of LSCM are:
1. Slow Speed
Each frame can take 100’s of milliseconds or more to capture. Slow acquisition rates negatively impact temporal resolution in living models and reduce productivity when imaging large sample groups for comparative analysis. In order to save time, the number of vertical slices can be reduced but results in the loss of valuable axial detail. Speed improvements can be made, but at the expense of a reduction in resolution or field size.
2. High Laser Powers
Typically, LSCM requires relatively high laser powers to produce good quality images. Imaging large volumes can result in photobleaching of the sample prior to completing the experiment. Again, this can be overcome, but by sacrificing the number of images in a volume. Similarly, when studying dynamic events laser power can perturb cell health impacting physiology and behaviour.
3. Low Dynamic Range
Fluorescent signals in samples range from very bright to very dim. LSCM’s detector technology has a relatively low dynamic range making it challenging to capture both bright and dim signal intensities in a single exposure. For example, acquisition parameters optimised to detect the lowest signal, result in the brightest signal being saturated. Similarly, acquisition parameters optimised to keep the bright signal in range, fail to capture low signal detail. Often two images are captured to overcome this problem, adding to workload and post-processing analysis.
Technology Solution
A camera-based confocal system can be a better solution for imaging larger samples, with fewer sacrifices. New developments in spinning disk confocal technology result in the required combination of large fields of view, high resolution, with lower laser powers, at much faster frame rates. For example, an image volume can be captured at least 10x faster at a like-for-like resolution when compared to a point scanning confocal, with less photobleaching or phototoxicity. Real-world examples include:
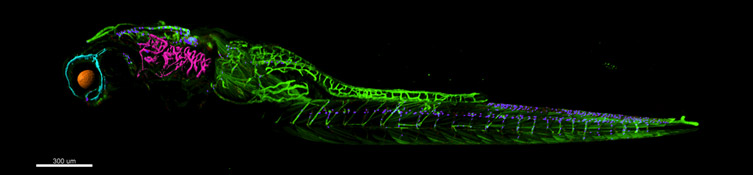
Andor’s Confocal Microscope Solution for Imaging Large Model Organisms

Andor strongly recommends the novel Dragonfly confocal equipped with either the iXon Ultra 888 back-illuminated EMCCD, or the Zyla 4.2 sCMOS cameras. Dragonfly has a unique confocal design which images at least as deep as a point scanner, with greater sensitivity and so richer image quality. It delivers a higher dynamic range meaning that both bright and dim signal can be captured in a single image. Its best-in-class low noise floor means you reduce the loss of weak signals. The culmination of these qualities results in better images for downstream visualisation and segmentation for analysis using 3D analysis software like Imaris
| Key Requirement | Imaging of Developmental Models Solution: Dragonfly |
| Maximising sample throughput of large specimens, retaining lateral and axial resolution | Using large area cameras and whole-field confocal illumination an instant high-contrast image is captured at real-time frame rates. Whole volumes comprised of 100’s of z-sections can be captured and displayed in seconds rather than minutes. Result 1 – No sacrificing field-of-view or lateral and axial resolution to save time. Result 2 – At least 10x faster throughput to reach publication sooner |
| Image through a volume minimizing photobleaching to maximise detail throughout | Dragonfly, with its spinning-disk multi-point design, Borealis excitation optics for field-optimised illumination, and sensitive cameras, delivers carefully targeted gentle illumination that minimizes photobleaching through a volume. Result 1– minimized loss of signal through a volume, safeguarding low signal detail. Result 2– no sacrificing image frames (axial detail) to avoid bleaching. |
| Image dynamic events at high frame rates preserving field of view and resolution | With its high-speed high-sensitivity cameras like the Zyla and Sona sCMOS the Dragonfly’s full field of view can be utilised and high-frame rates (up to 100 fps) achieved. This is achieved at full resolution. Faster frame rates are possible by cropping field size. Result – Highest temporal and spatial resolution whilst maintaining a full field of view for greater biological context. |
| Capturing both bright and dim signal from the sample without losing detail | Andor cameras on the Dragonfly all run at 16-bit, so offering the largest dynamic range available. The combination of Borealis illumination, the careful design of Dragonfly to minimise instrument noise, and the high sensitivity cameras mean you detect weaker fluorescence signals compared to other confocal platforms. Result – Capture all the detectable detail in your sample from the brightest to the dimmest in a single exposure. |
