Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Challenge Background
Calcium imaging is a widely used technique which can be used to study cell signalling in cell types such as neurons and cardiomyocytes. Fluorescent indicators such as fura-2, fluo-4 and genetically encoded calcium indicators can be used to directly visualise changes in free Ca2+ levels. The fluctuations in Ca2+ levels in response to various regulators can be detected using various fluorescence microscopy techniques and the profile of these responses subsequently analysed. This approach can therefore be used to help further our understanding of the myriad of interactions that affect the function and physiology of cardiac cells and in the screening of novel treatments for cardiovascular disorders.
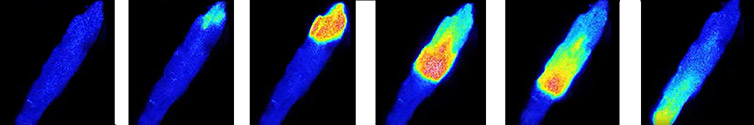
Imaging of live cell signalling processes is technically difficult: cell signalling events are by their nature very dynamic, with sometimes only very small changes in signal intensity needing distinguished against the background level. There may also the need to show the localisation of these events. In addition, the fluorescent markers themselves can affect normal cell physiology so they need to be kept at low loading concentrations making the need for ultra-sensitive detectors of paramount importance.
Technology Solution
EMCCD technology provides the ultimate sensitivity for the detection of the very weakest signals. EMCCD cameras can provide clear resolved images at signal levels that are below the detection limit of even the latest generation of sCMOS based cameras. EMCCD cameras are also capable of high speed imaging, with crop mode providing a further boost in temporal resolution.
Andor Camera Solutions for Cardiac Ca2+ Signalling Studies
Andor recommend the iXon Life EMCCD for calcium imaging in cardiac cells that typically use low-light optical sectioning modalities. The unsurpassed EMCCD sensitivity and superb custom ROI speeds make the iXon Life 888 and 897 EMCCD models the best possible detectors for temporally resolving fast calcium sparks and signal cascades, as well as small changes in low level signals that some studies require. The superb sensitivity offered by the iXon Life also means that dye concentrations can be minimized, reducing the ‘dye buffering effect’ that can distort the very physiology that is under study.
| Key Requirement | Imaging Cell Signalling of Cardiovascular Cells Solution: iXon Life |
| Detect and quantify Ca2+ dynamics at low signal intensities. | Single Photon Sensitivity and negligible read noise floor combine with > 90% QE to capture and register the majority of incident photons. Minimal thermal noise and Clock Induced Charge (Spurious Noise) results in the superb separation of background noise. Result – Enhanced photon detection and accelerated experimental throughput. |
| Measure dynamic events with superb temporal resolution | iXon Life 888 is the fastest EMCCD detector available, reading out at 93 fps at 512 x 512 and 26 fps for the full 1024 x 1024 array. Further acceleration is possible using user-defined sub-arrays, exceeding 600 fps from a 128 x128 sub-array. Result – Achieve superb spatiotemporal resolution of dynamic events. |
| Accurate cell physiology | iXon Life has exceptional sensitivity due to 95% QE and negligible read noise. This lets you reduce exposure times and fluorophore concentrations for more accurate cell physiology. Result – Obtain accurate physiological data. |
| Quality and Longevity | iXon Life comes with Andor’s exclusive UltraVac™ vacuum sensor enclosure. The well proven permanent vacuum process is critical not only for cooling, but for protection of the back-illuminated sensor against moisture and condensates. Result – Sustained high performance, year after year. |
References
Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Yvonne N. Tallini, Masamichi Ohkura, Bum-Rak Choi, Guangju Ji, Keiji Imoto, Robert Doran, Jane Lee, Patricia Plan, Jason Wilson, Hong-Bo Xin, Atsushi Sanbe, James Gulick, John Mathai, Jeffrey Robbins, Guy Salama, Junichi Nakai, Michael I. Kotlikoff. Proceedings of the National Academy of Sciences Mar 2006, 103 (12) 4753-4758; DOI: 10.1073/pnas.0509378103
