Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
When looking at biological tissues under a fluorescence microscope, the imaging depth is greatly limited by light scattering and absorption. To overcome this limitation, an increasing number of tissue-clearing protocols have been developed and improved in the last years. These techniques share the final goal of rendering biological tissues transparent by applying a series of chemical steps that equilibrate the tissue refractive index. Once the samples are clear, it is then possible to visualize whole organs and organisms in three dimension and with excellent detail even at centimeters in depth (Movie 1). For more information on this process and to choose the best clearing technique for your experiments, please read our technical article here.
Tissue clearing has drastically improved the way we look at biological tissues. However, there are still challenges when imaging this type of samples.
The first big challenge is time. Since cleared tissues allow imaging of much larger volumes, it is crucial to have a microscope that allows high quality imaging and is much faster than a regular point scanner confocal. Secondly, the system must be able to image deeper into the tissue without aberrations and with a good signal-to-noise ratio. The ideal microscope must also have a very flat and uniform illumination to avoid roll-off at the edge of each field of view and deliver seamless stitching of large, tiled images. Finally, the acquisition software should have the ability to easily render the three-dimensional datasets and to keep track of the imaging progress in real time.
Movie 1. Cleared mouse heart showing lymphatic vessels. Mouse heart cleared with the 3DISCO technique (1) and stained to show lymphatic vessels (Lyve-1-A488, cyan) and superior mesenteric arteria (SMA-Cy3, yellow). The confocal z-stack was acquired with an Andor spinning disk (Dragonfly) using an sCMOS camera Zyla 4.2 (New Model - ZL41 Cell). The image spans through 725 µm in thickness and is composed of 9 stitched tiles (3x3) taken with a 10X objective. Image credit: Claire Bouvard, Laboratoire BioSanté U Grenoble, France.
Andor's Dragonfly confocal is a multi-modal imaging platform that delivers unique capabilities to image cleared tissues. The multi-point scanning methodology used in Dragonfly (dual lens spinning disk) delivers high quality volumetric imaging at least 10 times faster than a point scanning confocal microscope. When paired with a high sensitive detector such as sCMOS Cameras, Dragonfly can deliver up to 400fps at 512x512 resolution.
Dragonfly also has an optimized pinhole pattern on the disk that reduces pinhole crosstalk and increases the background rejection, improving the image signal-to-noise ratio and allowing deeper imaging into thick samples. As shown in Movie 1, Dragonfly imaging performance has proven exceptional with thick specimens, routinely delivering high contrast in tissues and organs hundreds of microns thick.
Moreover, Andor's patented Borealis illumination delivers bright, uniform illumination across the entire field of view, which improves the stitching results in tiled montages (Figure 1). The Borealis system is coupled to a multi-mode squared fibre, which is more stable than single-mode fibres and can support excitation wavelengths of up to 800nm, and so is better suited to penetrate thick tissues.
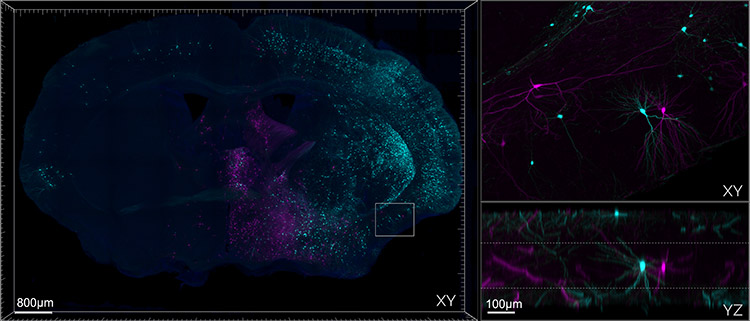
Figure 1. Mouse brain section cleared with SHIELD and Imaged on Andor Dragonfly. The left panel shows a montage of a 9 x 6 array of tiles stitched together, each one containing 245 z-steps, to cover roughly 500 µm in thickness. Top right: zoomed-in area showing the high quality of the image in the middle section of the slice. Bottom right: axial projection of the zoomed area. To prepare the sample, the mouse was injected with Rabies-GFP and Rabies-RFP, the tissue was SHIELD fixed, sectioned at 600um, passively delipidated with SDS, and stained passively with antibodies and DAPI. The sparse labelling of neurons is intentional as it allows for better tracing and identifications of the cells. Image credit: Hong Wei Dong, M.D., Ph.D. at UCLA, Department of Neurobiology.
The following table summarizes why Dragonfly spinning disk confocal is the microscope of choice to address cleared tissue imaging.
| Key Requirement | Solution for Cleared Tissue |
| Image large samples | Dragonfly is a fast confocal, at least 10 times faster than point scanning confocals. The internal beam splitter of the Dragonfly allows simultaneous acquisition of 2 channels with 2 independent cameras. Result - Acquire images at high speed up to 400 frames per second will increase productivity and throughput. |
| Image thick samples | Dragonfly's redesigned disk pattern improves the signal-to-noise ratio when imaging thick, cleared specimens. Result - Image through hundreds of microns up to the millimetre range axially with great z resolution and high contrast. |
| Homogenous illumination | Since cleared specimens are often large the uniformity of the illumination field is essential to obtain seamless tiled montages. Andor Borealis™ patented illumination technology offers even illumination across the whole field of view. Result - Acquire multiple tiles and build a montage. |
| Extended spectral range | The ILE laser engine paired with the patented Borealis™ illumination system supports a broad range of excitation wavelengths from the UV to the NIR (400-800 nm). Result - Use longer wavelength for deeper sample penetration. |
| Real-time 3D visualization and stitching | Fusion software utilizes Imaris 3D rendering for real-time visualization of the volumetric data. An integrates GPU accelerated stitching algorithm produces fast and seamless montage reconstructions. Result - Immediate visualisation of your data; build your stitched image while acquiring. |

Date: November 2022
Author: Anna Fasoli, PhD and Claudia Florindo, PhD
Category: Solution Note
