Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
“PI3 kinases – from basic cell biology to disease – pushing knowledge further.”
The endocytic and exocytic pathways are studied in the field of membrane-bound vesicle trafficking. The Endocytic pathway is of extreme importance for the homeostasis of the eukaryotic organism. Endocytosis is the formation of vesicles at the plasma membrane that are internalised into the cell and can travel to the cytoplasm. On internalisation, the vesicles can fuse to targeted organelles. Endocytosis is required (alongside other functions) to maintain active communication with extracellular signals, and for nutrient uptake. Research work done by Dr Kazuaki Yoshioka, Dr Khin Thuzar Aung and collaborators, that were led by Prof. Yoh Takuwa, has shown valuable insights into pinocytosis and vesicle trafficking (1, 2).
|
Glossary: |
|
|
HUVECs |
Human umbilical vein endothelial cells (HUVECs) |
|
PI3K |
phosphoinositide 3-kinases (PI3Ks) |
|
PI3K-C2 |
phosphoinositide 3-kinases (PI3Ks) class II |
|
SRRF |
Super-Resolution Radial Fluctuations |
|
Pinocytosis |
vesicular uptake of liquid extracellular material |
|
EE |
early endosomes |
|
CHC |
Clathrin heavy chain |
|
CLC |
Clathrin light chain |
Given the importance of the endocytic pathway to cell/organism normal function, it is not surprising that these processes are involved in the mechanistic pathways of numerous diseases, such as cancer, neurodegenerative diseases, diabetes, cardiovascular diseases, etc. Moreover, endocytosis can also be used as a valuable pathway for the delivery of therapeutic molecules. Therefore, understanding the mechanistic pathways of endocytosis is integral to enable the development of new therapies.
Mechanistically, endocytic vesicles are produced either by a clathrin-dependent process, by a caveolin-dependent process or by a clathrin/caveolin-independent process (3). Endocytosis can be divided into two different major classes: phagocytosis and pinocytosis. Phagocytosis is the engulfment of solid material by the cell; pinocytosis is the vesicular uptake of liquid extracellular material.
Molecules that are known to regulate the endocytic processes include polyphosphoinositides (PPIs). The phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that phosphorylate the inositol ring in of Phosphoinositides. PI3Ks act on the plasma membrane and in intercellular compartments (4). The PI3Ks control membrane lipid composition and regulate a wide range of intracellular processes, including vesicular trafficking and signal transduction (4). There are three major classes of PI3Ks: class 1, 2 and 3. These kinases are classified according to their phosphorylation specificity and in their structural differences. The least studied member of this family of Kinases is Class II PI3-Kinases (PI3K-C2). PI3K-C2 have been suggested to contribute to the pathology of diseases such as cancer and diabetes. As always in biology, when examined in detail, the level of complexity involved is high, and PI3 Kinases class II (PI3K-C2) have three different “flavours” (genes): PI3K-C2 α, β and ϒ. Current evidence suggests that the function of the different PI3K-C2 are not redundant (4).
In an earlier study, Dr. Kazuaki Yoshioka, Prof. Yoh Takuwa and collaborators showed that one of the PI3K-C2 kinases (PI3K-C2α) is essential for in angiogenesis and vascular barrier function (2). In this research, the author proposed that PI3K-C2α as a good target for cancer therapies since it´s inhibition resulted in lower microvessel density and overall volume of the implanted tumours (2).
In a recent study, Dr. Khin Thuzar Aung, Dr. Kazuaki Yoshioka, Prof. Yoh Takuwa and collaborators analysed how PI3 kinases regulate pinocytosis. Questions underlying key biological processes, such as which class of PI3 kinase regulates pinocytosis, what is the mechanism by which PI3K regulates pinocytosis, were tackled in this study. The researchers also tested the PI3 Kinase role in clathrin-dependent vesicle formation (1).
First, the authors tested which class of PI3 kinases is involved in pinocytosis. To answer this question, PI3K class I, II and III activity was inhibited, and the uptake of FITC-dextran vesicles was observed. The conclusion established PI3K-C2α and β as the kinases that regulate pinocytosis. Pinocytosis was shown to be partially mediated by clathrin- and dynamin-dependent processes in HUVECs (human umbilical vein endothelial cells). In fact, both PI3K-C2α and β are required for clathrin-mediated pinocytosis. These two kinases act by different mechanisms in the cell, since the inhibition of either completely inhibited clathrin-mediated pinocytosis.
To gain further insights into PI3K-C2α and PI3K-C2β, the authors used Dragonfly spinning disk microscope and super-resolution radial fluctuations - SRRF (5, 6, 7). Upon generation of fusion constructs, PI3K-C2α-GFP and PI3K-C2β-mCherry cells were transfected and imaged beyond the diffraction limit. PI3K-C2α-GFP was distributed as fine puncta throughout the cells, whereas PI3K-C2β-mCherry was enriched in the perinuclear region, in the peripheral regions surrounding podosomes and in the plasma membrane. A more detailed analysis allowed the conclusion that PI3K-C2β is closely associated with F-actin in the cell periphery, whereas PI3K-C2α is not. The significant different subcellular localisation of PI3K-C2α and β suggests that these two kinases could control different aspects of pinocytosis.
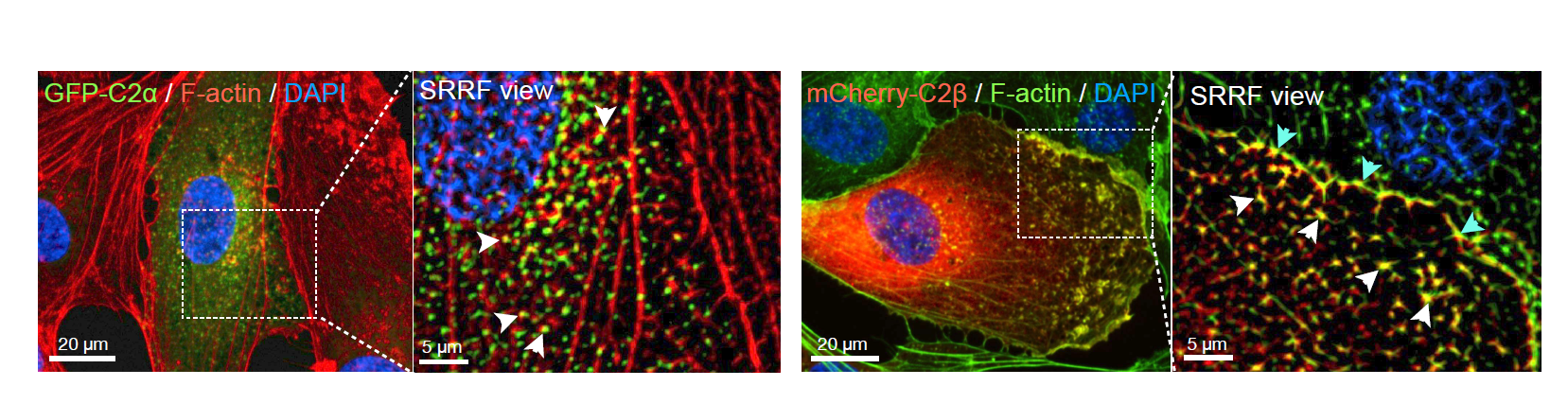
Figure 1: mCherry-C2β localizes with F-actin at the cell mebrane whereas GFP-C2α colocalizes with F-actin occassionally.
The specificity of PI3K-C2β in localising to F-actin prompt the authors to investigate the involvement of this kinase on the formation of pinocytic vesicle‑associated actin filaments. Dragonfly SRRF-stream imaging of dextran and clathrin light chain vesicles uptake upon RNAi of the PI3 kinases (C2 α and β), revealed the requirement of the C2β kinase in the formation of pinocytic vesicle‑associated actin filaments.
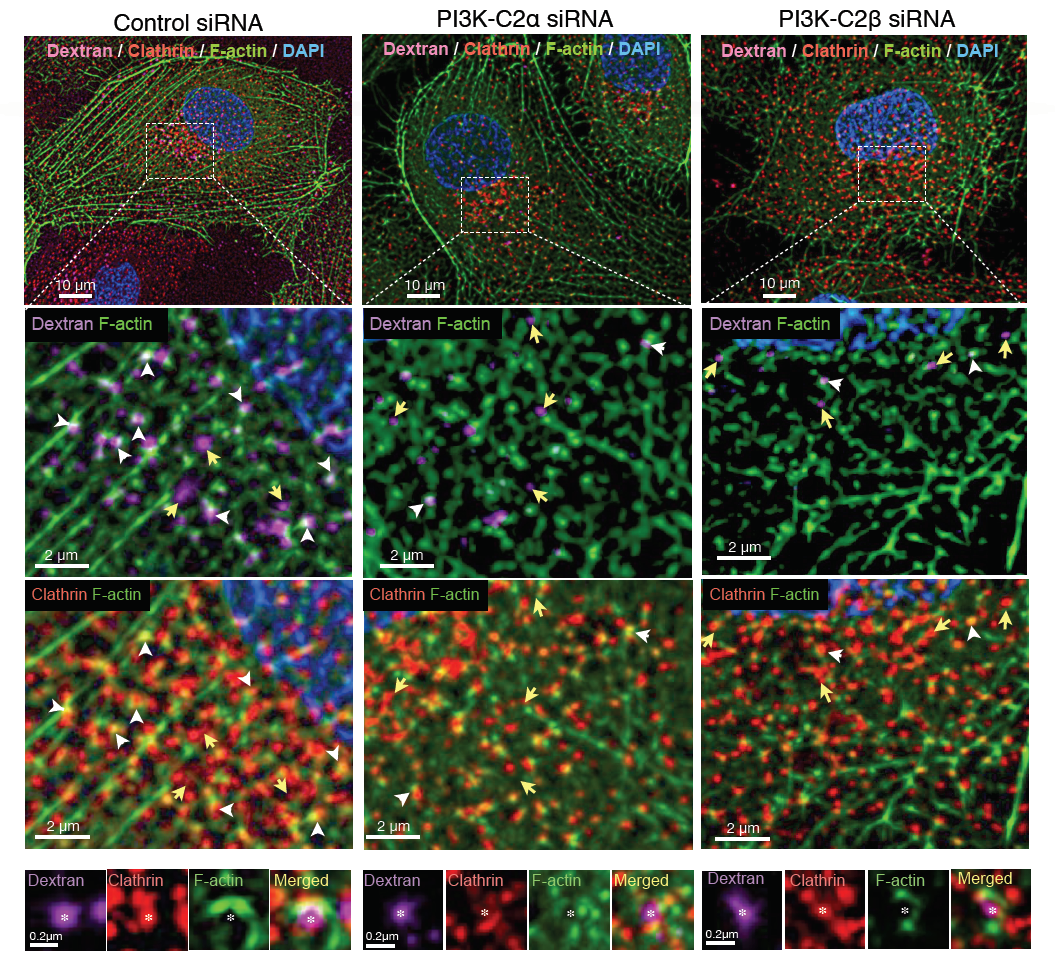
Figure 2: SRRF-stream and Dragonfly allowed the visulisation and conclusion that PI3K-C2β is required for the formation of pinosome-associated actin patches.
Intersectin-1 (ITSN1), is a multifunctional scaffold protein that binds to PI3k-C2β and localises in clathrin-coated vesicles. In order to better understand the mechanism by which pinocytosis vesicles are formed, the requirement for ITSN in the formation and function of clathrin-coated structures was investigated.
Depletion of ITSN1 by RNAi impaired the formation of F-actin structures (actin patches and stress fibres) and impaired the localisation of PI3k-C2β at actin patches and clathrin-coated structures. Therefore, the results suggested that ITSN1 is required for the formation of clathrin associated actin patches and for proper localisation of PI3K-C2β actin filaments-associated to clathrin-coated structures.
SRRF and dragonfly imaging allowed the discovery of the detailed localisation of PI3K-C2 kinases α and β, revealing the differences in their subcellular localisation (1,5,6,7). At clathrin-coated pits and/or vesicles: PI3K-C2β showed stronger co-localization with F-actin than did PI3K-C2α. PI3K-C2β was shown to be required for the actin organisation at dextran-containing, clathrin-coated structures.
In conclusion, the use of fast imaging at low illumination intensities with the Dragonfly, in combination with Andor cameras and SRRF-Stream mode allowed the authors to understand the mechanism of pinocytosis in human cells. The results showed that from the three classes of PI3 kinases, only PI3 Kinases class 2 are required for clathrin-mediated pinocytosis (1).
Still not convinced about the Dragonfly? Take a look at our interview with Dr Kazuaki Yoshioka.
Bibliography
Date: August 2019
Author: Dr Claudia Florindo
Category: Application Note
