Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Luminescence-based genetic reporter systems allow for exceptional sensitivity and are widely used to study gene expression in plants, bacteria and for in vivo studies of small animals. Systems using luciferase allow for the accurate quantification of very small changes in transcription within complex biological systems. In contrast to fluorescence, the signals involved are very weak. However, the background is inherently very low as autofluorescence that serves to interfere and mask the signal information when using fluorescence does not occur. Additionally, perturbation of normal cell processes due to illumination are also avoided. Luminescence can thus help to reveal transcriptional regulation in greater detail than is possible using other methods.

The iKon-M deep cooled CCD camera is widely used for luminescence imaging. In this example within a temperature-controlled light sealed chamber for plant imaging. Many other set-ups using high throughput plate format or microfluidic systems are also in use.
Bioluminescence studies provide many benefits but they do pose challenges from a detection perspective. These detection challenges are also quite different than for typical fluorescence microscopy-based imaging:
For luminescence experiments deep-cooled CCD cameras are the ideal solution. CCD cameras have lower dark current than the fast sCMOS cameras (by as much as 1000->fold). This can be made possible by deep-cooling the CCD to all but eliminate dark current, essential for these long exposures running into many minutes. CCD cameras have high quantum efficiency sensor options that allow for the widest response to a range of luminescence reporter systems. Some models also have high linearity required for quantitative measurements and wide dynamic range.
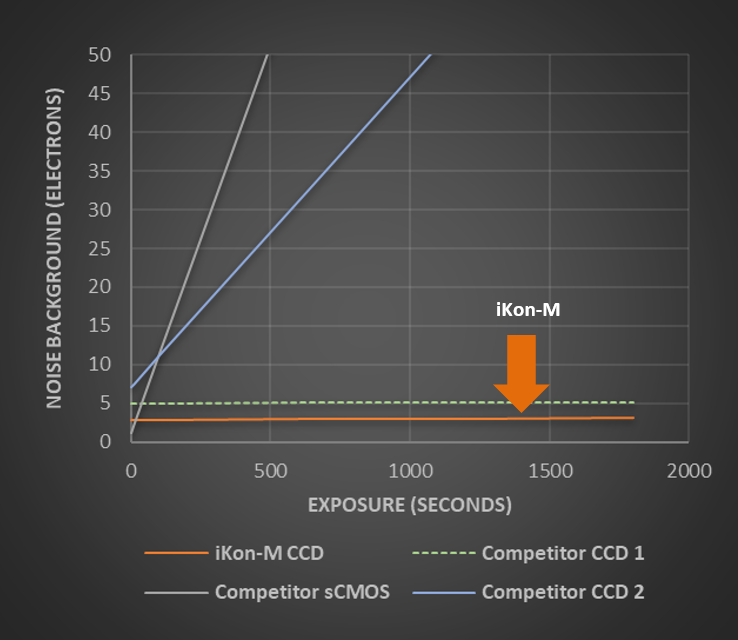
The iKon-M deep cooled CCD camera delivers the lowest background noise due to the combination of low read noise and the lowest possible dark current. This ultra-low background combines with high QE of >90% for the best possible sensitivity. The performance of the iKon-M CCD is especially apparent with longer exposures.
Andor’s iKon CCD camera series are the most sensitive CCD cameras available and are chosen for the most challenging luminescence experiments. Key to this industry best performance is the exclusive UltraVacTM permanent vacuum technology and efficient, stable thermoelectric cooling to -100°C. iKon CCD cameras are able to drive dark current down to the lowest possible levels, which combines with up to 95% Quantum efficiency to deliver the highest sensitivity. A c-mount fitting makes the iKon-M CCD model flexible whether coupling to a microscope, or to a lens as part of a custom optical set-up.
| Key Requirement | Luminescence Studies Solution: iKon-M |
| Detect very weak photon emission signals from luciferase activity | With over 90% QE across the emission wavelength range of most luciferases, the iKon-M CCD camera captures every photon possible. A 13µm pixel size provides a balance of photon collection and high resolution in a 1024x1024 sensor format that fills the industry standard c-mount lens format. Result – Enhanced photon detection and accelerated experimental throughput. |
| Extended time measurements with exposures of minutes | With industry leading cooling down to -100°C, the iKon-M provides the lowest possible dark current. Even for long exposures of 20-30 minutes, it is possible to achieve the lowest possible noise floor and best possible signal to noise ratio. Result – achieve the best separation of the signal against the lowest possible background |
| Record accurate physiology | The superb sensitivity combines with the highest quantitative accuracy. This makes it possible to uncover even subtle effects at the transcription level such as due to circadian rhythms. Result – Have confidence in your data. |
| Quantify weak and high-level signals in one image | With a signal handling capacity of up to 130,000 e- a wide dynamic range ability is assured. Low level information can be quantified simultaneously with high level signals. This has a practical benefit in experimental design allowing wide signal levels within the same experiment. Result – Obtain accurate physiological data in one single image. |
| Quality and Longevity | iKon-L comes with a 5-year guarantee on the UltraVacTM vacuum sensor enclosure. The well proven permanent vacuum process is critical not only for cooling, but for protection of the back-illuminated sensor against moisture and condensates. Result – Sustained high performance, year after year. |
When moving from typical luminescence experiments towards studies at the single cell level, even higher sensitivity may be required. For such studies we recommend the iXon EMCCD series: find out why in our Single Cell Bioluminescence Solution Note.
Date: Nov-20
Author: Dr Alan Mullan
Category: Solution Note
