Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Chinese hamster ovary cells are commonly used for industrial applications such as the production of recombinant proteins for therapeutic use. The original cell line was first established in 1956 in the laboratory of Theodore Puck (Eleanor Roosevelt Institute for Cancer Research) with the definitive type being CHO-K1, and further variants appearing over time. At one point in time given the lack of other mammalian cell lines as a useful model research model, it was described as being a mammalian version of E.coli. The CHO cell line is well characterised, and its genome has been sequenced. CHO cells have a low chromosome number which has helped to make them easier to work with for studies of genetics. These factors all help to ensure the CHO cell lines continues to remain one of the most widely used cell lines.
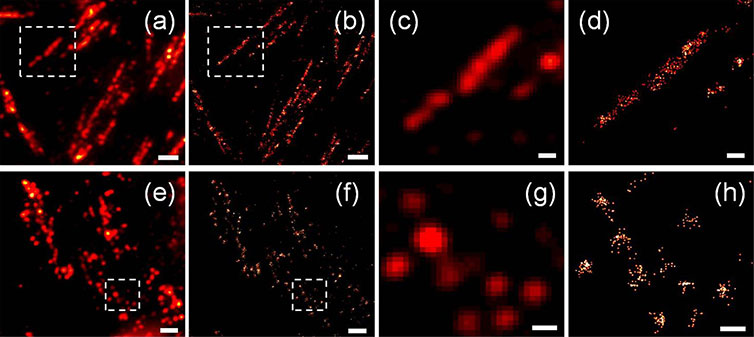
Fluorescence images of focal adhesions in CHO cells on the homogeneous (a–d) and 400 nm fibronectin patterns (e–h). The summed fluorescence images are displayed in panels (a), (c), (e), and (g). The reconstructed super-resolution images are displayed in panels (b), (d), (f), and (h). Panels (c), (d), (g), and (h) show the magnified areas indicated in the boxed areas of panels (a), (b), (e), and (f) respectively. The scale bars are 2 μm in (a), (b), (e), and (f), and 500 nm in (c), (d), (g), and (h).Courtesy of Courtesy of Fan-Ching Chien, Department of Optics and Photonics, National Central University, Taiwan; Peilin Chen, Research Center for Applied Sciences Academia Sinica 128, Nankang, Taiwan, Research Center for Applied Sciences Academia Sinica 128, Nankang
The widespread use of the CHO cell line is due to several factors. First, it is easy to grow in a suspension culture as well as a monolayer. Moreover, CHO can be grown in controlled media of known chemical composition, which ensures batch reproducibility between cell cultures, allowing for controlled experiments. CHO can be modified via post-translation to recombinant proteins with human biocompatibility. High yields of recombinant protein per cell can be achieved using optimised chemical selection and gene amplification systems specifically for CHO cells making it perfectly suited for commercial applications.
Date: May 2020
Author: Dr Alan Mullan & Dr Aleksandra Marsh
Category: Application Note
