Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Scientists can learn about the functions of specific neurons in complex networks, like the brain using proteins that act as “photoswitches”. Optogenetics has proven highly effective in neuroscience allowing for greater precision than previous electro stimulation approaches. Light can turn photoswitches on and off in a highly controllable way, enabling precise control of activity in specific cells and thus permit observation of how processing and behaviour are altered by defined neuronal populations in circuits and wider tissue.
Processes happen fast in neurons, so speed is a critical parameter for many studies. The bacteriorhodopsin and HaloR families are light activated inhibitory proteins that allow for millisecond resolution. They have and will continue to be very useful, however they are not without room for improvement. Both require continuous illumination to stay activated, and continuous light can cause other problems such as rebound excitation, or eventual partial inactivation. Also, HaloR can require high light intensities because the magnitude of the current is driven by the constant activation of the pumping cycle.
Thus, researchers led by Ehud Y. Isacoff from the University of California, Berkeley and the Lawrence Berkeley National Laboratory designed a ligand-gated ion channel that is K+ selective and controlled with light. Called HyLighter, the ion-channel isn’t active until exposed to light, is more sensitive to light than HaloR, and can reach its maximum current over a wide range of light intensities.
The light-activated ion channel can convert a light pulse into a stable hyperpolarizing current that stays on with no illumination until it is turned off using a complementary wavelength. It is based on an ionotropic glutamate receptor (iGluR). These cation channels mediate excitatory neurotransmission in higher organisms and are permeant to both Na+ and K+.
To create HyLighter, the researchers first engineered chimeric iGluRs with the ligand-binding domain of iGluR6, the photoswitched tethered ligand attachment site that encodes light sensitivity in the excitatory LiGluR channel, and the pore of sGluR0, an exotic bacterial iGluR homologue. They experimented with the various chimeras they created and found that one had all the characteristics they needed. It functioned as a light-gated K+ channel, was maximally activated by low light intensity at wavelengths ~390 nm, maintained activity in the dark, and could be turned off with 500 nm light.
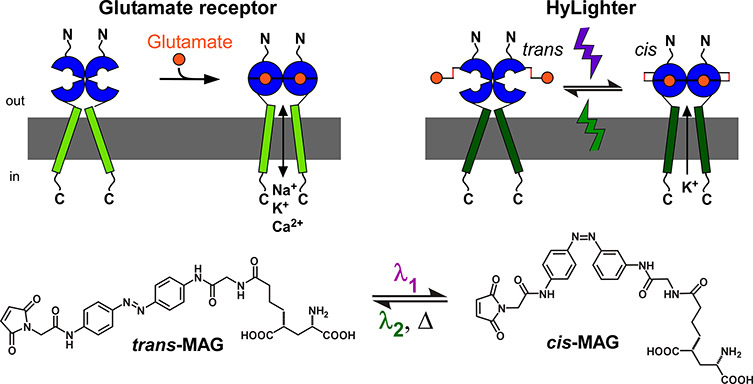
Figure 1. An ionotropic glutamate receptor (top left) and a synthetic photoswitch called Maleimide-Azobenzene- Glutamate (MAG; bottom) is the basis for the light-controlled HyLighter ion channel (right). Images courtesy of Dr. Harald Janovjak.
They tested HyLighter by transfecting cultured hippocampal slices from early postnatal rats with HyLighter-GFP using biolistic gene transfer. It was expressed well in all regions of the hippocampus and homogenously distributed in all parts of the neuron. They activated HyLighter with 390 nm illumination from a Lambda DG-4 light source coupled to the microscope, and projected onto the sample through the Mosaic through a 40x objective.
“Among the existing microscopy solutions, only the Mosaic allows you to continuously illuminate an arbitrary mask in the imaging field. You simply can’t do this using laser-scanning microscopes, which never provide true simultaneous illumination. This important feature combined with ease of use and direct interfacing with software makes the Mosaic a unique and valuable tool for the optogenetic community,” said Dr. Harald Janovjak, who was part of the research team (now at the Australian Regenerative Medicine Institute, Monash University).
In the hippocampal slices, HyLighter induced strong hyperpolarization that could silence neuron firing until deactivated by 500 nm light. At the sample, the light intensity was approximately 20 mW mm-2 at 390 nm and 40 mW mm-2 at 500 nm.
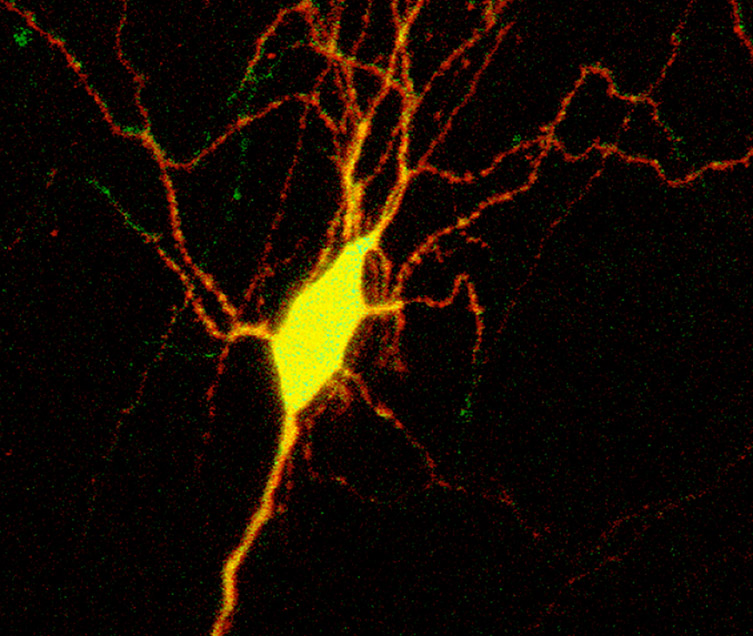
Figure 2. MIP image of overlayed confocal stacks HyLighter:GFP with non-specific tdTomatoe. Note the cell shows widespread (yellow) presence of HyLighter GFP, which can be used to silence the neuron when pulse illuminated with 390nm. Pulse illumination at 500nm inactivates the K+ channel.
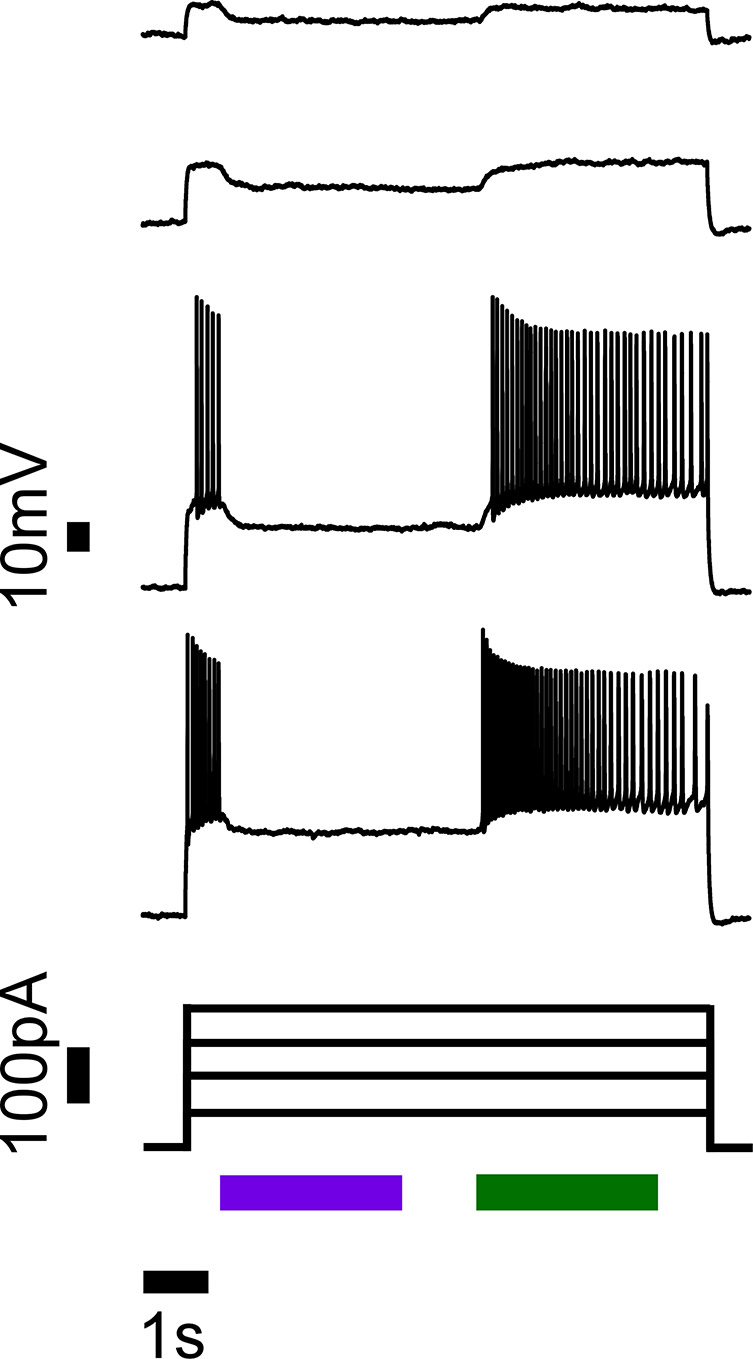
Figure 3. Action potentials triggered by current injections are robustly silenced when HyLighter is activated by 390 nm light (violet bar) and no longer inhibited when deactivated with 500 nm light (green bar).
The researchers also generated transgenic zebrafish model expressing HyLighter. When the tails of these fish received 390 nm, the probability of an escape response after a mechanical stimulus was reduced. This was reversed by 500 nm illumination. The illumination had no effect on escape responses in zebrafish not expressing HyLighter. The researchers say that as a new, light-activated, purely hyperpolarizing ion channel, HyLighter complements existing optogenetic tools. Its push-pull two-wavelength design, low light requirement and unique spectral sensitivity make it a useful way to suppress activity in specific cells in intact neural circuits with temporal precision. In addition, the two-wavelength design means that it can be used in experiments in which neurons are silenced after light exposure and behavioural analysis then performed in ambient light or in the dark without a visual stimulus effecting the results.
“When it comes to silencing of nerves cells, bi-stability and the requirement for small amounts of light make HyLighter unique,” said Dr. Janovjak.
HyLighter and other native and synthetic optical highlighter proteins that have followed have gone on to help address many key problems in neurobiology.
Appreciation is gratefully extended to Dr. Harald Janovjak, Australian Regenerative Medicine Institute, Monash University and Dr. Ehud Isacoff, University of California, Berkeley.
Date: December 2021
Author: Dr Mark Browne and Dr Alan Mullan
Category: Application Note
