Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
One in five adults in the UK is deaf or has some degree of hearing loss. Hearing impairment results in communication difficulties and can lead to an increase in social isolation and depression. The COVID-19 pandemic has disproportionately affected those with hearing loss, as facemasks and social distancing have made communication even harder. In addition to this, some studies suggest an association between hearing loss and dementia, where mild hearing loss could double the risk of developing dementia, while this risk increases to five times in those with severe hearing loss (learn more).
Hearing loss has several underlying causes; these include age, noise, genetics, disease, and certain types of therapeutic drugs. Drugs that cause ear impairment are known as 'ototoxic' (ear-toxic) and one class of these drugs is the aminoglycoside antibiotics. Aminoglycosides are used to treat life-threatening bacterial infections such as sepsis and multidrug-resistant tuberculosis but, as an unfortunate side effect, they damage the sensory hair cells of the inner ear causing hearing loss and balance disorders.
The hair cells of the inner ear are so called because they have fine hair-like projections called stereocilia at their apical end that move in response to sound vibrations. Hair cells transform the mechanical motion of the stereocilia into electrical signals that our brain interprets as sounds (such as speech and music). In humans and other mammals, hair cells are not capable of regenerating, so once lost they can never be replaced. Hearing loss is therefore irreversible, and for this reason, research in this area is of high importance.
In this article we present:
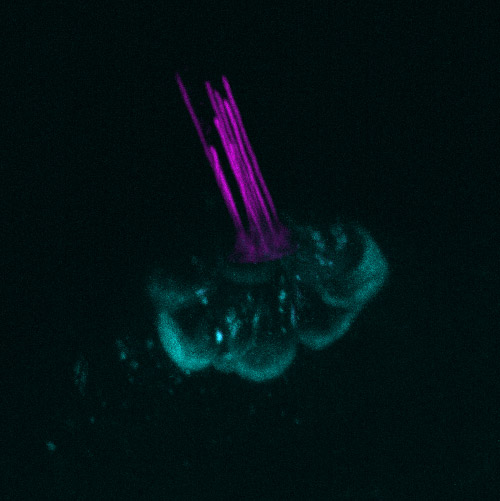
Figure 1 - Zebrafish posterior lateral line hair cells from pou4f3 GFP transgenic larvae. Hair cells express green fluorescent protein (GFP) in cyan and are in the process of loading with Texas-Red Gentamicin (TRG) in magenta. The TRG has been added to the media the larvae are maintained in and at this time point (3 minutes post addition of TRG) only the kinocilia and stereocilia are labelled with TRG. Cells were imaged using Andor Dragonfly. A) Maximum intensity projection (MIP) of the hair cells stacks B) 3D rotation of the hair cells stacks. MIP and 3D visualisation of the hair stacks assembled with Imaris software.
Research in polarised epithelial cells requires various experimental methods, and light microscopy plays a significant role among them.
In order to visualise intracellular transport in these cells, the imaging system must allow gentle live imaging coordinated with high-speed image acquisition. A highly sensitive system with exceptional background rejection is essential. In addition, the system should deliver imaging on a wide variety of scales, from subcellular structures to tissue, organ, or organism level (Figure 1 shows imaging of the hair cells in a live zebrafish larva), that will allow the researcher to understand how subcellular dynamics impact the tissue, organ or organism.
Andor’s Dragonfly includes exceptional features that are ideal for polarised epithelial imaging. Its high acquisition speed and real-time 3D rendering allow for rapid imaging and visualization without any loss in resolution. Imaging up to 400 frames per second can be achieved with the system. For an overview of these cells within the organ or organism, the large field of view combined with Borealis uniform illumination deliver seamless stitching, allowing on-the-fly visualization on every scale.
We asked Dr. Kenyon why Andor’s Dragonfly is a crucial tool to understand intracellular transport in sensory hair cells?
Dr. Kenyon replied that:
"The Andor Dragonfly has been invaluable for this [study of transports in hair cells], particularly as transport of these aminoglycosides in zebrafish hair cells happens over a short period, requiring fast image acquisition."
"Further, the dyes used to identify the intracellular compartments of the cell are particularly prone to photobleaching, something that's hugely reduced using the Dragonfly."
Figure 2 - Outer hair cells in an organotypic mouse cochlear culture. Hair cells are labelled with LysoTracker green in cyan and Texas Red Gentamicin in magenta. Cells were imaged using Andor Dragonfly. The 3D rotation of the hair cells was executed using Imaris software.
Dr Emma Kenyon is an Independent Research Fellow funded by the Royal National Institute for Deaf People (RNID). Dr Kenyon is based in the Kros/Richardson laboratories in the Neuroscience Department within the School of Life Sciences at the University of Sussex. The laboratories are interested in understanding how the inner ear works and develops. The main goal is to unravel the leading causes of deafness and balance disorders.
Dr Kenyon’s research concentrates on how life-saving ototoxic drugs lead to deafness and balance disorders. More specifically, she focuses on the mechanisms by which the aminoglycoside antibiotics (e.g., gentamicin, kanamycin) damage sensory hair cells. The aim of Dr Kenyon’s research is to protect hair cells from these ototoxins and to preserve the hearing of patients in need of these drugs.
| Glossary | |
| Aminoglycoside Antibiotics | Aminoglycoside antibiotics are broad spectrum antibiotics used to treat Gram-negative bacteria and can be used synergistically with other antibiotics against Gram-positive bacteria. |
| Gentamicin | Gentamicin is an Aminoglycoside antibiotic. |
| Kanamycin |
Kanamycin is an Aminoglycoside antibiotic. |
| Organotypic culture | In-vitro culture of an organ collected from an organism. |
| Ototoxins | Ototoxins are molecules that cause ear impairment (otoxic = ear-toxic). |
| Stereocilia | Stereocilia are hair like projections on the apical surface of the sensory hair cells of the auditory and vestibular system essential for hearing and balance. |
Dr Kenyon’s research is specifically interested in intracellular transport in sensory hair cells and how aminoglycosides are transported once inside the cells. To answer her questions, Dr Kenyon has conjugated a fluorophore (Texas Red) to a selection of different aminoglycosides. These are then tracked in the sensory hair cells of the zebrafish lateral line and in mouse cochlear cultures using fluorescence microscopy. The zebrafish lateral line contains hair cells similar to those in the mammalian inner ear (cochlea) but as they are located on the surface of the fish it is possible to image them using fluorescence microscopy in a live animal without additional tissue preparation. The zebrafish lateral line and mouse cochlear cultures are complementary models for this research, but they come with challenges:
Dr Kenyon’s experiments involve fluorescent confocal imaging of Texas-Red conjugated gentamicin in the hair cells of live zebrafish larvae or mouse cochlear cultures. Hair cells are labelled with dyes that target intracellular compartments and images are taken at set time points. In the zebrafish model, hair cells are imaged every five minutes for up to 80 minutes (Figure 1), while with mouse cultures images are taken at 2, 4, 16 and 24 hrs (Figure 2 and 3). These experiments allow tracking the movement of the gentamicin and visualise which intracellular compartments they may be trafficked to and when.
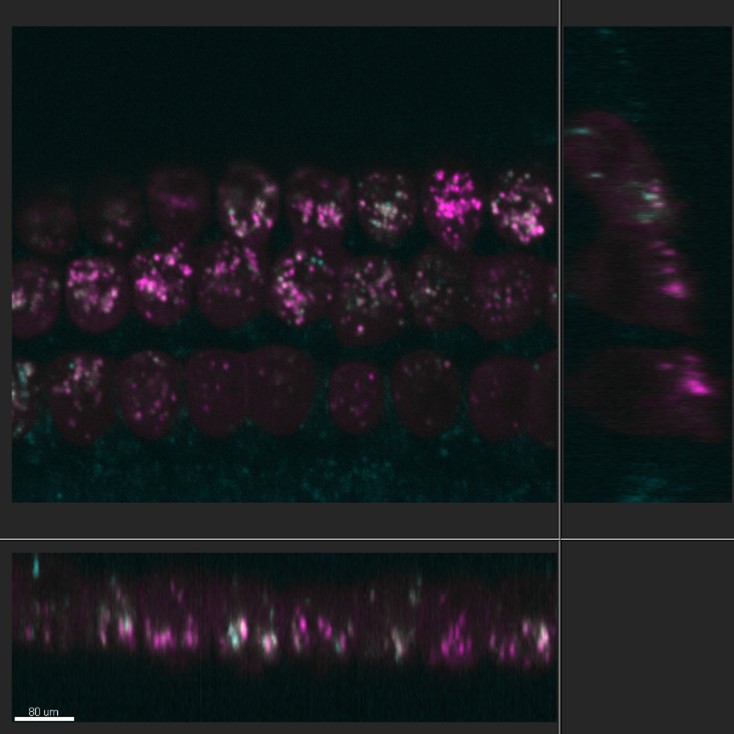
Figure 4 - Orthogonal cross-section through the outer hair cells of an organotypic mouse cochlear culture. In order to analyse the hair cells Dr Kenyon takes advantage of the Imaris software's orthogonal view to visualise in all planes the distributions of Texas-Red gentamicin when compared with the distribution of LysoTracker green. Hair cells are labelled with LysoTracker green in cyan and Texas-Red Gentamicin in magenta. Cells were imaged using Andor Dragonfly.
We have asked Dr. Dr Kenyon a final comment about ANDOR Dragonfly:
"I love how quickly the system starts up and shuts down, making it ideal for on-the-fly experiments."
This project is funded until the end of 2021.
Date: September 2021
Author: By Claudia Florindo & Dr Emma Kenyon
Category: Application Note
