Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Calcium ions are known to play a role in many aspects of cell function. One of the functions of intracellular Calcium is a role in cell development, as the cell passes through the different stages of the cell cycle. One study that has helped provide insights in this subject was that of Professor Mike White and Dr. Violaine Sée at the University of Liverpool. In this work they used a targeted flash photolysis approach that enabled the role of calcium in the progression of the cell cycle to be studied.
D-type cyclins such as CD1 are known to play an important role in cell cycle progression. Levels of CD1 vary during the cell cycle with the level directly dependent on transcription rate. Transcription of CD1 itself is regulated by multiple transcription factors, including nuclear factor kappa B (NF-κΒ), which can control cell growth and differentiation through transcriptional regulation of CD1. Even though NF-κΒ appears to play a role in cell cycle progression, the intracellular events leading to this transcription factor’s activation upon growth factor stimulation were unclear. Stimulation of non-dividing cells with growth factors or mitogens can be seen to cause a transient rise in intracellular free Calcium concentration. Intracellular calcium has also been shown to play a role in the G1/S and G2/M phases of the cell cycle, but the exact role of Calcium in the transition from G0 to G1 was not known.
By using targeted photolysis to uncage a Calcium chelator and a calcium donor in living cells White and Sée were able to observe how the cells responded to precisely-timed absence of (normally-present) calcium signals and increases in calcium levels. This allowed them to understand the role that Calcium plays in CD1 gene transcription and subsequent cell proliferation.
To study the long-term role of the serum-induced intracellular calcium increase, the research team needed a noninvasive method to eliminate this transient increase. To achieve this they used the caged calcium scavenger Diazo-2. Its normally low affinity to calcium increases 30-fold when illuminated at 360 nm. To eliminate a serum-induced calcium peak, they applied the illumination to Diazo-2-loaded cells just after serum induction. The Diazo-2 photolysis then inhibited the serum-induced Calcium peak without effecting subsequent intracellular calcium levels.
The researchers used the MicroPoint system to uncage Diazo-2 in specific cells of interest by exposing these cells to a sequence of 4 ns flashes of 360 nm light over 10 s. They performed simultaneous imaging and photostimulation of the specimen and could therefore follow the targeted cells and normal cells with time-lapse confocal microscopy. In this way they directly monitored the effects of uncaging on intracellular signaling and cell fate.
As Violaine Sée explained, they did not detect NF-κΒ translocation into the nucleus or NF-κΒ-dependent gene transcription from the NF- κΒ consensus and CD1 promoters in the targeted cells. These facts suggested that the serum-induced Calcium peak appears to signal the down-stream events.
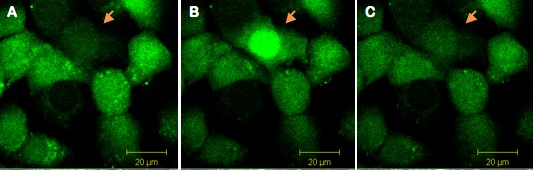
Figure 1. Single cell illumination of Swiss 3T3 cells loaded simultaneously with NP-EGTA (caged Calcium) and Fluo-4 (calcium dye). The arrow indicates the illuminated cell (A) before illumination and (B) during illumination. The increase of fluorescence indicates calcium release in the illuminated cell. (C) Thirty seconds after illumination, Calcium levels are back to basal levels.
To further study the relationship between the transient Calcium increase and later NF-κΒ activation, they performed targeted photolysis of the caged Calcium donor NP-EGTA. Activated NP-EGTA causes a 22% transient increase in intracellular calcium. This increase is of the same magnitude as the serum-stimulated increase, but it did not induce detectable p65 translocation to the nucleus.
The result of suppressing and replicating serum-induced Calcium peaks using caged molecules suggested that NF-κΒ translocation and downstream regulation of gene transcription depends on a transient increase of intracellular calcium, but other serum-dependent signals are required to activate NF-κΒ. These results, combined with other experiments, led the researchers to propose that the G0–G1 transition depends on mitogenic kinase activity as well as calcium signaling and that NF-kB activation has a critical role in the transduction of these mitogenic signals to the nucleus.
“The work showed that calcium has a lead role in the cell cycle. Calcium ions have been implicated in many aspects of cell function, but its role in the cell cycle was not well described,” said Violaine Sée. “We found that calcium can initiate the cell cycle through activation of the mitogen-activated protein kinase–NF-κΒ pathway. -Dr. Violaine Sée
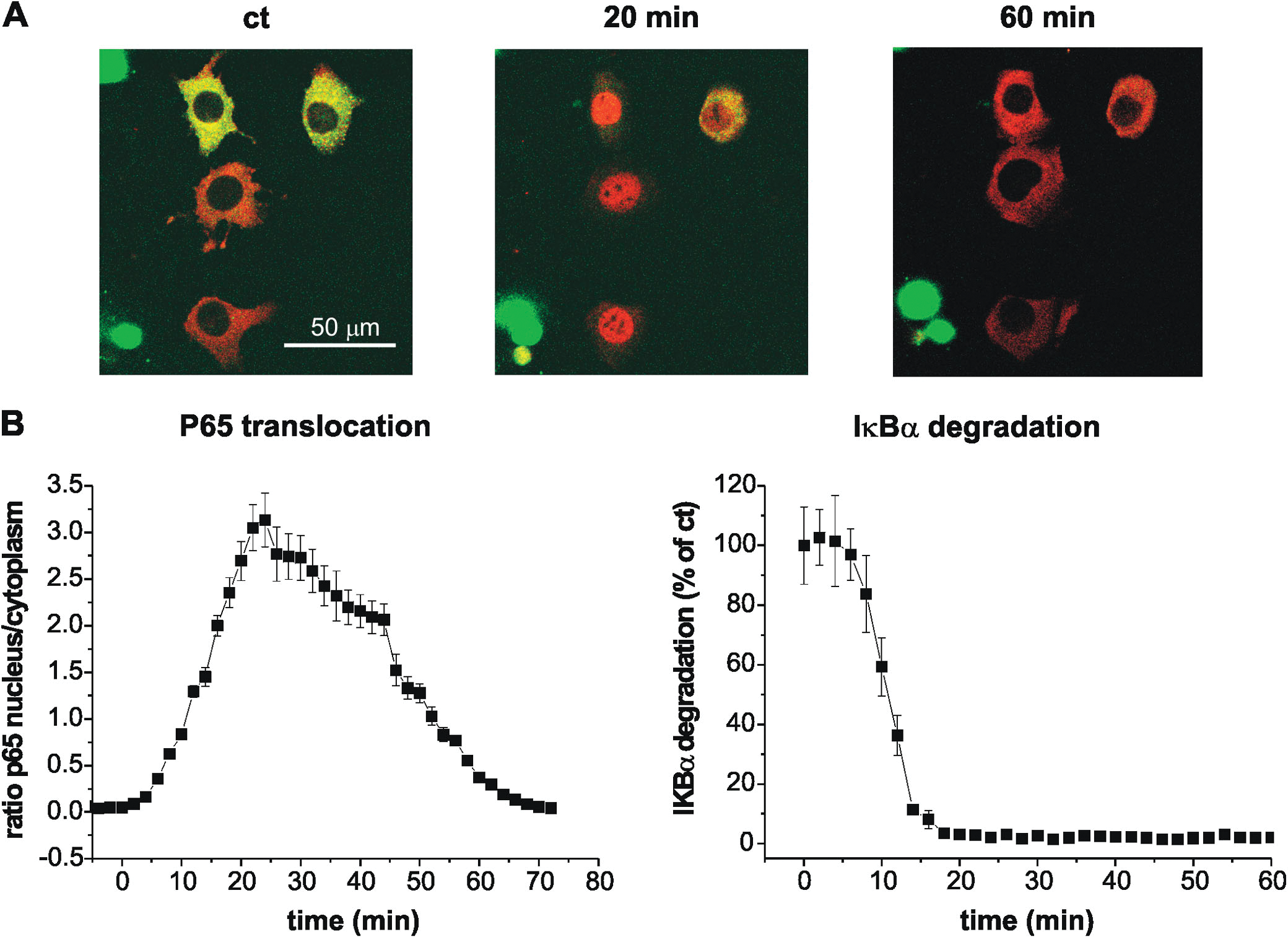
Figure 2. Serum stimulation promotes p65 translocation into the nucleus as well as IκBα degradation.
Images used courtesy of Dr. Violaine Sée, University of Liverpool. Thanks to Professor Mike
White and Dr. Violaine Sée, and Dr. Dave Spiller (now of University of Manchester).
Related Reading
Date: Dec 2021
Author: Dr Mark Browne and Dr Alan Mullan
Category: Application Note
