Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
The organization of a cell is critical for its function and understanding how organization affects function is a major goal of cell biology. One of the fundamental questions is how cells use the cytoskeleton to integrate spatial information into cell cycle regulation. One study of note is that of a research group led by Professor John A. Cooper (Head of Biochemistry and Molecular Biophysics at the University of Washington) and Dr Alexey Khodjakov at the Wadsworth Center, Albany, New York that used microtubule ablation techniques of various components of the cell during the cell cycle.
Budding yeast cells are one of the common model organisms used to study cell division. During division in the budding yeast, Saccharomyces cerevisiae, one end of the mitotic spindle is drawn through the bud neck to deliver a genome to the daughter cell. Cytoplasmic microtubules projecting from the spindle pole bodies carry out this process by interacting with the cell cortex to orient the spindle along the mother-bud axis and then move one spindle pole body through the neck and into the bud.
The cell has quality control mechanisms in place in case this process goes wrong. For example, mutations can cause a delay in the movement of the spindle. In this case mitosis proceeds in the mother cell, but if things have not corrected themselves by anaphase a cell-cycle checkpoint mechanism known as the spindle position checkpoint will stop mitosis. Scientists have some understanding of how this checkpoint halts mitosis, but it isn’t fully known how the checkpoint mechanisms detect that the spindles are not in the correct position to proceed. Previous work had suggested that dividing yeast prohibit cell cycle progression when the mitotic spindle is not adequately positioned between the nascent mother and daughter cells. This implies that the cell must monitor the position of the spindle, and interpret it relative to some other site, such as a landmark.
To test the hypothesis that cytoplasmic microtubules extending from the spindle to the bud neck are important for this process Cooper’s team interrupted microtubule interactions with the bud neck using laser ablation. The researchers used a MicroPoint pulsed laser system with its emission tuned to 539 nm to perform laser microsurgery of GFPlabeled microtubules in dynein mutant budding yeast cells. These dynein mutants cannot pull the spindle through the bud neck, and thus mitosis is stopped by the spindle position checkpoint.
Often the MicroPoint system is used for optogenetic experiments as a way to add a photobleaching / photoactivation module to a microscope, but the tunability of the system’s laser also gives the flexibility to also use the system for ablation as was done for microtubules in this experiment. MicroPoint allows the user to image the specimen during laser ablation and this allowed the researchers to visually target dynamic microtubules for ablation. Microtubules move quickly through the cell, so being able to observe the cell throughtout ablation was critical for nimble targeting and subsequent verification of microtubule severing.
After using pulses of 539 nm light to sever individual cytoplasmic microtubules between the bud neck and spindle pole bodies, the researchers observed displacement of a distal fragment from the remaining microtubule. The fragment and remaining microtubule depolymerized and then grew back after several minutes. They monitored the spindle of these cells for up to 90 minutes to see if the cells remained arrested in anaphase. For imaging, they took timelapse Z series of the cells using an inverted fluorescence microscope with a 100 x, 1.35 N.A. oil objective lens, spinning disk confocal with 488 nm laser excitation and an EMCCD camera for detection. The Z series covered 3 µm in depth and the time series were captured at 30 or 60 s intervals.
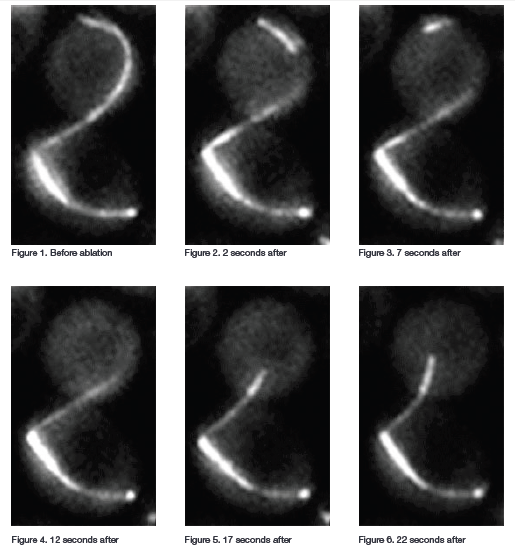
Figure 1: Mitotic Spindle before Ablation, Figure 2: 2 seconds post Ablation, Figure 3: 7 seconds post Ablation, Figure 4: 12 seconds post Ablation, Figure 5: 17 seconds post Ablation, Figure 6: 22 seconds post Ablation.
When cytoplasmic microtubules in the bud neck were ablated most of the cells exited mitosis, showing that the absence of cytoplasmic microtubules in the bud neck activates the mitotic exit network. The researchers next performed experiments to find out if disrupting cytoplasmic microtubules not in the neck would disrupt the checkpoint. In checkpoint-activated cells with microtubules extending from one spindle pole body through the neck they ablated microtubules at the other spindle pole body (microtubules not extending through the neck). Most of these cells remained in mitosis. Finally, they did experiments in which they damaged the spindle pole bodies or severed microtubules near the spindle pole bodies. Neither of these actions prompted mitotic exit.
“Collectively these ablation experiments show that disrupting the microtubules that extend from the spindle to the bud neck cause the cells to fail to prohibit cell cycle progression and thus to exit mitosis. This suggests that the cytoplasmic microtubules are important for reading the position of the spindle upstream of cell cycle regulation”.
These findings have implications across species. They may be particularly important for the faithful distribution of genomes to proper regions of the cytoplasm during the asymmetric cell divisions that underlie metazoan development and the homeostasis of adult tissues.
Appreciation is gratefully extended to Professor John A. Cooper, University of Washington and Dr Alexey Khodjakov, Wadsworth Center, Albany, New York
Date: December 2021
Author: Dr Mark Browne and Dr Alan Mullan
Category: Application Note
