Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Photoactivatable fluorescent proteins (PA-FPs) can undergo dramatic changes in their properties in response to illumination of a specific wavelength. In basic terms they can be converted from a dark inactive state to a fluorescing, active state. As with uncaging, there are a multitude of applications that this can be used for, most notably studying a specific dynamic cell process within the wider cell. Some of the main forms of PA-FPs are summarized as follows:
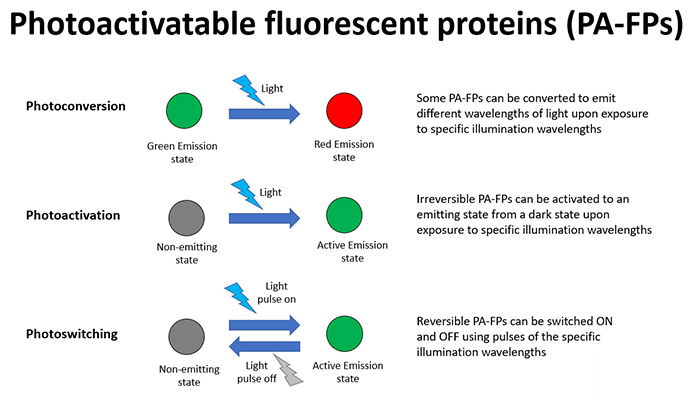
The highly targeted effects that these techniques provide mean that there are a multitude of possibilities for optical labelling and tracking cells and any organelles of interest. Important intracellular molecules can also be observed and studied within cells where their general abundance can prohibit the understanding of a process in a localised region of the cell.
In order to perform these light-mediated experiments we need to control the delivery of illumination to the specimen in a tightly controlled manner. This includes control of illumination power and wavelength and also the timing of this illumination to region(s) of interest.
Mosaic and MicroPoint are two products that are designed to suit such photostimulation experiments, each having some benefits that suit particular usage scenarios.
Mosaic is a digital mirror-based array (DMD) that can be combined with light sources - from LED, arc lamps to higher power lasers including MicroPoint.
What Mosaic allows is the total flexibility in illumination of the specimen. This means different shapes and sizes of illumination areas can be defined- even in complex, or multiples of different regions of different shapes and/or patterns of illumination. Since exact timing is kept across the full field of view using this technology, along with a range of triggering options, highly complex experiments are possible, as well as boosting productivity in each experiment. This makes Mosaic a highly effective way to perform photoactivation based experiments on your microscope.
MicroPoint is an illumination source for photostimulation applications. It uses a pulsed nitrogen pumped tunable dye laser system.
MicroPoint is widely used to provide higher power densities for use in DNA or cell damage and repair studies as well as uncaging. However, it can be used at lower levels of illumination such as for photoactivation, making it a very flexible tool. Illumination from the MicroPoint can be directed to points of interest in the cell to activate PA-FPs with the fine degree of time control these experiments can demand.
Date: February 2023
Author: Dr Alan Mullan
Category: Solution Note
