Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
The development of green fluorescent protein (GFP) and its wide application in biological imaging has provided a rich legacy of tools for transfection of genes across species. These techniques have also given rise to a wide range of optically active cell lines and transgenic animals. Proteins such as Opsins like Channelrodopsin-2 expressed in these engineered cells and organisms, respond in precise ways to specific, targeted illumination of defined wavelengths. This capability, seen by many as the dawn of optogenetics, has been integrated into the toolsets of many research groups tackling the many questions of cellular processes.
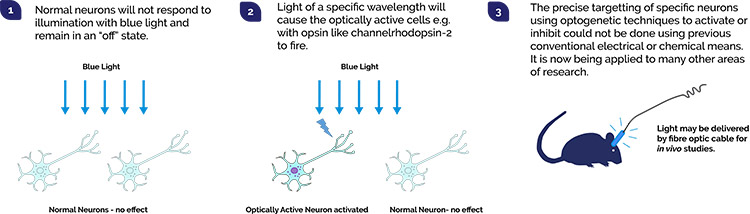
As more light responsive proteins (optogenetic actuators) and techniques are developed, ever more researchers are considering how they may make their imaging system capable of exploiting the optogenetics toolset. One of the difficulties is how to control illumination delivery to the specimen, so that the right illumination power, of the right wavelength reaches the desired place, at precisely the right time.
| Type | Optogenetic Protein | Peak Excitation (nm) | Effect |
| Channelrhodopsins | ChR2 | 470 | Activation |
| Chrimson | 590 | Activation | |
| ReaChR | 590 | Activation | |
| Halorhopsins | NpHR | 570 | Inhibition |
| Jaws | 632 | Inhibition | |
| Archaerhodopsins | Arch | 566 | Inhibition |
| ArchT | 566 | Inhibition | |
| Leptosphaeria rodopsins | Mac | 540 | Activation |
Table 1: List of Light Responsive Proteins and key attributes. Note that many variants of each of these examples have been engineered with different properties such as response of action and shifting wavelengths to more biologically gentle, longer wavelengths.
There are two main approaches for controlling illumination generally used for optogenetics and optophysiology studies:
1. Beam steering (“Galvo”) – the illumination may be steered by galvanometer (or other optical means) in x and y to scan across the specimen. This permits fine control of illumination power from low to higher powers. The wavelength used can be changed as required e.g. By exchangeable, cost-effective dye cells. Using this approach there is typically limited control the illumination area parameters: size, shape and one area at a time only.
2. Digital Micromirror Device (DMD) within the optical path is coupled with an LED or laser illumination source. The DMD is comprised of a high-resolution array of mirrors- each of which can be switched on to reflect light, or off to deflect light out of the light path. Since there is control of each individual mirror element it is possible to create regions of illumination from the simple to the complex, or even cycle illumination patterns. It is also possible to have multiple regions with the same time synchronization that is not possible using beam steering. Areas outside illumination are protected from exposure of illumination (and photodamage).
Another less common way to achieve precise illumination is by Holographic Projection. This method does allow customization of illumination pattern, but is much less widely used due to higher costs and difficulties integrating into a typical microscope and may have performance issues such as uniformity of illumination.
Mosaic is built around a DMD array specifically configured for the requirements of optogenetics experiments. Since it is DMD based, there is great flexibility for a wide range of experiments. Multiple regions of interest can be illuminated simultaneously and precisely as required. Complex user defined “masks” can easily be configured using the onboard memory for user-created ROIs, or over wider areas of the specimen. This makes Mosaic the most suitable for the widest range of optogenetics based experiments. Wide and custom illumination regions are simply not possible, or certainly more limited, using a galvanometer-based laser steered approach. The Mosaic is compatible with a broad range of LED and laser light sources over a broad wavelength and power range.
Use Mosaic with your microscope and illumination source such as MicroPoint, or as part of a Dragonfly Confocal System and benefit from optogenetics techniques in your experiments.
MicroPoint is a “galvo based” illumination system optimised for photostimulation applications. It uses a pulsed nitrogen pumped tunable dye laser system. It is often used to provide higher power densities for use in DNA or cell damage and repair studies, bleaching experiments like FRAP or FRET and uncaging of bioactive molecules. Illumination can be directed to localised areas, however it cannot be applied to larger groups of cells or complex areas of the specimen as is possible with mosaic.
| Parameter | MicroPoint | Mosaic |
| Light Source Compatibility | MicroPoint is a laser based system (laser dye cells used to provide required wavelength). EPI illumination can be added. | Compatible with many light sources ie. LEDs, arc lamps, Lasers |
| Wavelength Range | Can be configured with laser dye cells from 365 to 656 nm. An effective and low-cost way to add wavelengths to the system. | 360-880nm |
| Resolution | Near diffraction limited – dependent on microscope objective | Near diffraction limited – dependent on microscope objective |
| Multi-region Illumination | No – illumination beam is scanned across the sample in a point-by-point basis | Yes- array has full flexibility in illumination pattern and complexity/multiple ROIs. |
| Integration into Microscope Systems | Easily integrated with most modern upright and inverted microscopes | Two models allow integration to both upright and inverted microscopes from the major manufacturers |
| Compatible with Dragonfly Spinning Disc Confocal? | Yes | Yes |
| Application Suitability | MicroPoint has a wide application suitability for many applications from uncaging, through to ablation experiments. The limitation of MicroPoint is in less freedom in illumination patterns. You cannot have multi-regions of illumination or complex patterns with the same timing as the illumination is scanning across the sample. | The broadest and most flexible solution for photostimulation experiments. The limitation for Mosaic is that it does not provide high power illumination for experiments like ablation and cutting applications. |
To find out more about optogenetics and photostimulation, please see our webinar presented by Dr Mark Browne Introduction to Photostimulation. This webinar covers the history and key developments in optogenetics and applications from micro-surgery and molecular diffusion to optogenetics control of cells and organisms.
Date: February 2023
Author: Dr Alan Mullan
Category: Solution Note
