Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
From the first stages of cell division through to the maintenance processes of the fully developed organism, repair processes are continually responding to various forms of damage. Quite understandably there is a need to better understand these vital repair pathways of the cell. There are many topics to consider: what impacts the ageing process, how do neurons regenerate, cancer prevention and improved treatments, repair of blood vessels, tissues, and wound healing. Although we have characterized some of the main pathways used, for example, those involved in DNA repair, and other specifics like how vesicle trafficking is repurposed for repair of cell membranes, there is still much more to uncover and understand. To study the repair pathways involved it is very useful to have an effective way of inducing damage to the component of the cell in question.
One way we can do this is by using focused illumination. Short pulses (picosecond or nanosecond in duration) of highly focused laser light can be used to precisely target regions of interest in cells. Appropriately designed laser pulses can result in localized damage, breaking of chemical bonds and photoablation. Under the right conditions, a localized plasma forms within the illuminated volume and molecular bonds break more quickly than they can reform: material is then damaged, or even evaporated. This approach provides the means to perform cutting, ablation or induce damage, where and when we want it. DNA, for example is typically targeted with pulses of 365 or 405 nm. The labelled components that may be involved in the repair process can then observed under fluorescence microscopy.
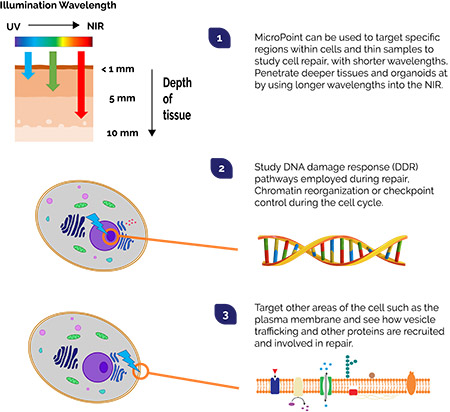
"Micropoint is a flexible and field-proven tool for performing ablation based on a pulsed nitrogen pumped tuneable dye laser system. It has been used in a diverse range of experiments for studies of DNA damage and repair, plus many other studies of the wider cell repair processes. It is also possible to use MicroPoint for many other optogenetics based experiments from photobleaching for FRAP, to uncaging bioactive molecules”.
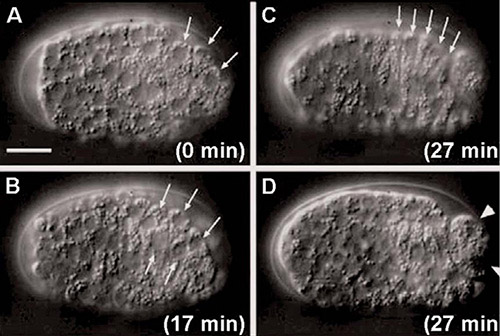
Figure 2: Ablation of Muscle precursor cells performed with MicroPoint in developmental studies of C.elegans.
Use MicroPoint with your microscope, or part of a Dragonfly Confocal System and add photostimulation capabilities to access optogenetics techniques.
| Feature | Benefit |
| Patented compact, pulsed nitrogen-pumped tuneable dye laser | ✔ Effective and proven for use in many DNA and cell damage and repair studies ✔ Let’s you control illumination to the region of interest where and when you need it |
| Broad wavelength range of 365 to 656 nm | ✔ Use short wavelength UV pulses for thin tissues, cells or embryos. ✔ Longer wavelengths access deeper tissues. ✔ Adapt photostimulation conditions while optimizing imaging for sensitive specimens. |
| Precise and controlled Energy Delivery | ✔ Perfect for ablation and bleaching at higher power densities ✔ Perform experiments that require lower illumination levels such as uncaging and photoactivation. |
| Flexible configuration: More than 20 wavelengths possible using dye resonator cells, select from range of dichroic filter sets | ✔ Remarkable flexibility to configure for your specific experiments ✔ Have simultaneous imaging and photo-stimulation of the specimen |
| Range of UV-Vis imaging quality Epi illumination adapters available | ✔ Add photostimulation techniques to your existing or new microscope. ✔ MicroPoint can be added to most current and previous generation microscopes from leading microscope manufacturers |
Find out more about MicroPoint.
