Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Model systems in developmental biology use a variety of transgenic animals in which fluorescent proteins label individual cells, particular tissues or whole embryos. Such fluorescent transgenic organisms offer the opportunity to visualize cell and tissue behaviour during development processes of high resolution and, in, real time, observations that might shed light on the dynamics that are involved in shaping a complex organism. This is often limited by the technical constraints of the imaging apparatus. In-vivo imaging can potentially capture quantitative data at a single-cell resolution. When this imaging is performed non-invasively on intact, fully functioning organisms, time lapse microscopy allows the study of development over time.
Fluorescence microscopy techniques have become increasingly powerful in terms of resolution, speed and penetration, they most often only do so in thin and transparent samples. The size and opacity of whole embryos, which are often a few millimetres in size, make it especially challenging to achieve single cell resolution. In addition, the resolution that can be achieved in live specimens is generally lower than in fixed specimens because of the size of the sample, the scattering of intact and opaque tissue, pigmentation in untreated animals, the movement of living organs (skeletal muscle, gut, heart, blood, eyes etc) and the need to keep the sample under physiologically sustainable conditions. Due to these limitations, scientists are forced to fix and section their samples, even though many of the highly dynamic processes that occur during development can only be studied in full detail in the intact living embryo.
SPIM is a fluorescence microscopy technique that uses a focused light-sheet to illuminate the specimen from the side. SPIM achieves excellent resolution at high penetration depths while being minimally invasive at the same time. SPIM offers a number of advantages over established techniques such as strongly reduced photo-bleaching, high dynamic range, and high acquisition speed. These features make SPIM a popular technique for studying development.
The idea behind SPIM and other light-sheet-based microscopy techniques is to illuminate the sample from the side in a well-defined volume around the focal plane of the detection optics. Even though there are many different implementations of this idea, the general principals are the same.
In SPIM, cylindrical optics are used to create a sheet of varying thickness. The light converges towards the sample and diverges away from it. The waist of the light sheet is positioned in the centre of the field of view. The dimensions of the light sheet can be adapted to different sample sizes: for smaller samples (20-100 um), the light sheet can be made very thin (~ 1um); whereas for larger samples (1-5 mm), the sheet has to be thicker (~5-10 um) to remain relatively uniform across the field of view. What is significant with SPIM is that only the in-focus part of the specimen is exposed to laser light, which provides optical sectioning and substantially reduces photodamage. Moreover, the fluorescence signal emitted from the in-focus section is detected in parallel for the entire field of view, which provides high imaging speeds.
Optical penetration depth is fundamentally limited in light-sheet microscopes by light scattering. To overcome this obstacle simultaneous multiview light sheet microscopy has been developed. This technology eliminates spatiotemporal artifacts caused by slow sequential multiview imaging and introduces powerful new capabilities. Simultaneous multiview SPIM can rapidly image large fluorescent specimens from multiple directions over biologically relevant timescales using sCMOS technology from Andor. This technique overcomes the limitations of sequential multiview strategies and enables quantitative systems-level imaging of fast dynamic events in large living specimens.
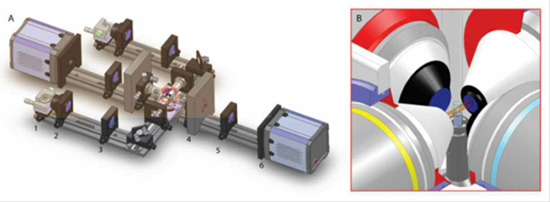
Figure 1: Schematics of selective-plane illumination microscope with two illumination and detection directions, Courtesy of Lars Hufnagel, EMBL, Heidelberg.
Andor Solutions for SPIM
The full range of sCMOS cameras from Andor are ideal detectors for SPIM. The Neo 5.5 and the Zyla 5.5 have a 5.5-megapixel sensor that offers a large field of view and high resolution without compromising read noise or frame rate. The Zyla 4.2 has a 4.2-megapixel sensor with a QE of 72 % at 600 nm. The sCMOS cameras from Andor have a small pixel size of 6.5 micrometers which ensures sufficient oversampling of the pointspread-function, even for objectives with low magnification (e.g. 10x NA 0.3; 20x NA 0.5). The read noise is very low, even when compared with the highest-performance slow readout CCDs. The Neo 5.5 and Zyla 5.5 can achieve a read noise down to 1 e-, while the Zyla 4.2 has a read noise of 0.9 e- , all without signal amplification, while reading out 5.5 megapixels at 30 & 100 frames per second respectively for the Neo 5.5 and Zyla 5.5 and 100 fps for the Zyla 4.2, thereby facilitating the live acquisition of dynamic events which take place during development.
| Product | Description | |
|---|---|---|
| Neo & Zyla sCMOS | Scientific CMOS (sCMOS) is a breakthrough technology that offers an advanced set of performance features that render it ideal to high fidelity, quantitative scientific measurement. The 5.5 megapixel sensor offers a large field of view and high resolution, without compromising read noise, dynamic range or frame rate. Rolling and Global (Snapshot) shutter readout ensure maximum application flexibility. | Learn more |
| Andor SDK3 | The Andor Software Development Kit (SDK) gives the programmer access to the Andor camera and spectrograph range. The key part of the SDK is the dynamic link library which can be used with a wide variety of programming environments including C, C++, C#, Visual Basic and LabVIEW. |
