Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
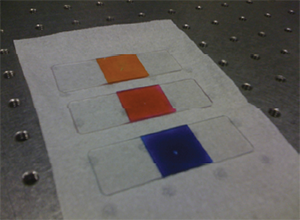
Figure 1: A digital photograph of three different concentrated dye solutions sandwiched between a glass slide and a coverslip. From top to bottom: Na-FITC (0.5 g/mL), Rose Bengal (0.3 g/mL), Acid Blue 9 (0.35 g/mL).
Written below is a simple protocol detailing a method to create a fluorescent test sample that can be used to measure the illumination uniformity of your confocal microscope (laser scanning or spinning disk). This protocol is an extension of a procedure originally developed by Michael M. Model at Kent State University involving the formation of concentrated fluorescent dye solutions [1, 2]. As a result of their large optical density, these dye solutions possess the unique property that, if sandwiched between a glass slide and a coverslip, they will only emit fluorescence from an extremely thin, planar region (diffraction-limited in the axial z-direction) immediately adjacent to the coverslip when illuminated with an appropriate excitation wavelength. Therefore, when viewed with a confocal microscope, any non-uniformities in the fluorescence image of the concentrated solution reflect the degree of non-uniformity of the illumination profile for a given set of aligned optical components in the confocal microscope (laser, filters, objective, image forming optics, pinhole(s) and detector). Different dyes can be selected for different excitation/emission wavelength combinations. These concentrated dye solutions are preferred over traditional fluorescent plastic slides for this type of measurement because the plastic slides accentuate the pinhole cross-talk effect due to their intense brightness and thier thickness that extends well beyond the focal volume.
Materials
| Dye | Excitation Wavelength | Emission Wavelength | Sigma-Aldrich Product code |
| Sodium-Fluorescein (Na-FITC) | Blue (473 nm, 488 nm, 491 nm) | Green (510 nm - 560 nm) | F6377-100G |
| Rose Bengal | Green(532 nm, 543 nm, 561 nm) | Red (560 nm - 620 nm) | 198250-5G |
| Acid Blue 9 | Red (633 nm, 642 nm) | Deep red (650 -800 nm) | 861146-5G |
Protocol
Image Analysis
The degree of illumination uniformity can be quantified with any commercial image processing software package such as Metamorph (Molecular Devices) or ImagePro (Media Cybernetics). One can also download and use the freely available open-source image analysis program ImageJ. The instructions described below are a step-by-step guide for uniformity analysis in ImageJ, but the same general method can be applied in any of the commercial software packages as well. Alternatively, Spectral Applied Research can produce and email you a detailed report about your system’s illumination uniformity, free of charge (see instructions below for transferring data to Spectral).
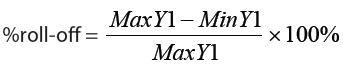
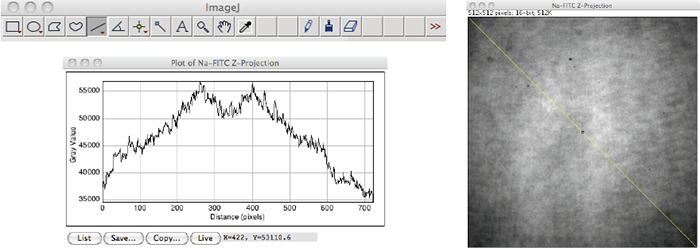
Figure 2: A Z-Projection image of a Na-FITC concentrated dye solution z-series with a drawn top-left to bottom-right line segment (left) and the corresponding line profile graph of pixel intensity values along this line (right). Image acquired with a Yokogawa CSU-X1; 491 nm excitation; 525/30 nm emission. Analysis performed with ImageJ version 1.46.
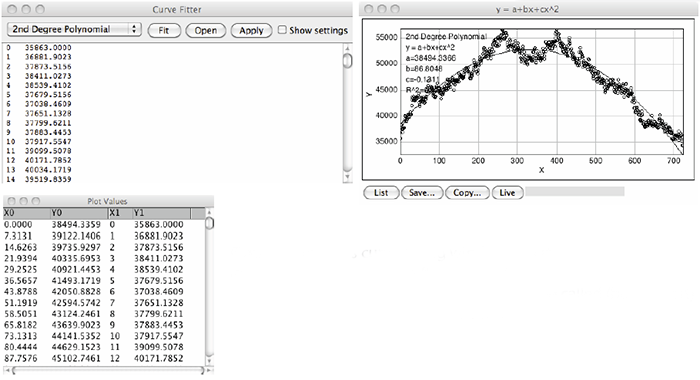
Figure 3: ImageJ’s curve fitting window tool (top left) and a resulting fitted line profile graph (top right). By clicking the List button in the graph window, a Plot values table will be called (bottom left) from which the MaxY1 and MinY1 values can be determined.
References
