Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
It is well understood that the positioning of genes within the cell nucleus is not random and plays a role in certain nuclear functions. It has been postulated that expression for each gene is confined to certain regions within cells, however the understanding for how and why this occurs remains poorly understood. The significance of gene territories within living cells can potentially provide greater understanding for how overall gene expression is affected by gene location within the cell nucleus. This question has been posed by Olivier Gadal from Université de Toulouse
and his fellow investigators at the Pasteur Institute in Paris. Dr. Gadal and his colleagues propose that the spatial organization of genome is by no means random, and represents a significant role in genomic transcription, regulation, DNA repair and regulation.
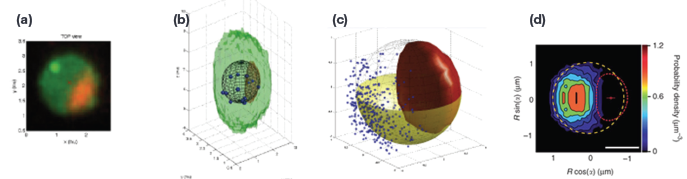
Figure 1a - To substantiate gene expression territories, Fluorescence image sets were acquired via an Andor Revolution Spinning Disk Confocal (Andor, Belfast Ireland) and three dimensional maximum intensity projections were created. Locus and nuclear pores are green and the nucleolus in red. Selection of interphase cells occurred through both manual and automatic detection methods. Manual methods were foirst employed, and then to reduce operator induced variability, automatic detection of interphase nuclei was performed. Using the MatLabderived routine Nucloc (nucloc.org).
Figure 1b - Resulting regions of interest were processed to first extract 3D loci coordinates, nuclear centers, nucleolar centroids, nuclear envelope ellipsoid and nucleolar volume. The collected data points were further evaluated through custom-developed quality control procedures designed to correct for chromatic shift and to eliminate objects that did fall within measurement parameters used to define the theoretical nucleolar volume extents.
Figure 1c - shows an example 3D view of locus positions (green spheres) obtained from approximately 2,500 nuclei, after nuclear landmark alignment; red spheres indicate nucleolar centroids; yellow hemisphere radius, 1 μm; the red surface shows the 'median' nucleolus.
Figure 1d - Probability maps for resulting measurements were then created. Nuclei from Fig. 3 are shown as a heat map in Fig 4. The median nuclear envelope is indicated by the dashed circle, the median nucleolus by the dashed red curve, the median location of the nucleolar centroid is indicated by the small red circle4. Additional statistical sampling is used to confirm locations and placements.
To substantiate these observations Gadal observed the formation of GFP-labeled coregulated galactose and bioregulated biogenesis genes territories in the mCherry-labeled nucleoli of living Saccharomyces cerevisiae yeast cells. Previous observation of gene territories has been performed, however the observations were obtained from fixed cells, and in two dimensions. Gadal has chosen to observe live cells in a three-dimensional format, which allows more complete and dynamic probabilistic mapping of the sub-nuclear gene territories in question. Central to his observation of cells are three important factors. The first is overcoming the present limitations of fixed cells and the lack of spatial detail required to better predict the probabilistic location of gene territories. Of equal importance is overcoming the physical limits of resolution based upon traditional light microscopy techniques; gene compartment sizes within a eukaryotic nucleus are generally accepted to be only slightly larger than the 500 nanometer axial resolution and 250 nanometer lateral resolution available from typical light microscopes. A final consideration was the need to automatically acquire large numbers of images to more accurately assess gene territories. Stochastic motion within the nucleus requires large populations for study, hence the need for a large number of observations. And because automated methods are employed in the experiment to select nuclei within interphase cells, it is important to have the most highly resolved image sets possible.
Gadal turned to Andor's Revolution Spinning Disk Confocal Microscope (SDCM) to address current limitations in conventional light microscopy and obtain the necessary high performance required to collect the number of images needed to accurately predict gene territories. In doing so, he addresses the need to maintain the viability of living cells over repeated exposures, collect the required number of observations to make accurate predictions of gene territory areas or regions while simultaneously addressing the resolution requirement needed to automatically select and observe gene loci within their respective domains.
As in many live cell imaging applications, speed of imaging while maintaining highly resolved (both intensity and spatial) objects is the primary requirement. Living cells visualized through fluorescent tags are prone to phototoxic and photobleaching effects. A microscope’s optical path provides a certain degree of assistance in managing a limited light budget, but cannot account for all the factors affecting image acquisition. High numerical objective lenses allow more in-focus light to be detected and electron-multiplying CCD cameras effectively amplify that signal while suppressing background noise. The inclusion of a spinning disk assists in the further improvement of signal to noise ratio, while simultaneously creating a more gentle and photostable imaging environment.
Speed of Imaging
Live yeast cells are used to better understand the probabilistic locations of the coregulated galactose and bioregulated biogenesis genes. While previous experiments have determined gene location through fixed yeast cells, a more highly resolved map is the ultimate goal of this research. Stochastic motion within live cells provide that environment, however, the cells must be treated carefully to properly view and map gene locations. The apertures in the spinning disk confocal provide a much gentler imaging environment than traditional widefield or laser scanning techniques, whilst meeting the resolution requirement necessary to visualize subresolution detail.
Resolving Sub Resolution Detail
It is commonly accepted that lateral resolution within the light microscope environment is approximately 0.25 micron, with axial resolution commonly accepted to be approximately 0.5 micron. The yeast cell nuclei in these experiments are estimated to be 1.0 micron spheres, impressing important dynamic considerations on highly resolved sub-cellular features. It is possible through the use of high numerical aperture objective lenses to restrict the depth of field issues that lead to loss in resolution, however, the addition of the spinning disks pinhole apertures provides a much more highly resolved series of images from which to perform the automated analyses required of the probabilistic gene territory maps.
Collecting Large Amounts of Data
To accurately predict gene locations, it is necessary to collect a large number of yeast nuclei. In this instance approximately 2000 cells were acquired for each expressed gene and then images automatically processed to include only those in interphase and expressing the gene of interest. In addition to traditional scanning, the third dimension of depth is taken into account. For each population of cells scanned, 41 axial images are first acquired to visualize the cells' entire volume and then to create the maximum intensity projection used to automatically find nuclei in interphase. Automated nuclei detection requires a stable and repeatable imaging environment. Without it, automation would not be possible, requiring more manual methods, which have been repeatedly shown to reduce experimental objectivity and accuracy.
The work of Dr. Gadal further refines existing research findings that describe the location and extent of gene territories within eukaryotic cell nuclei, and does so over longer time periods than previously reported. It is revealed through his research that gene territories in question are smaller than anticipated, which in turn constrains the interactions of genes with "each other, the nucleolus or the nuclear envelope." The findings show that positions of genes within the nucleolus are important, as those positions help define whether a gene is activated or repressed. It is through these observations that the role of nuclear architecture is far more important than previously assumed. The role of spinning disk microscopy helps reveal these arrangements and makes these observations possible.
