Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Light can be produced by matter which is in an excited state and, as we will show, excitation can come from a variety of sources. The atoms and molecules that make up matter typically emit light at characteristic energies. The light emission can be spontaneous or stimulated.
In spontaneous emission, matter at a sufficiently high energy level can relax by emitting photons of a characteristic energy - this is the process that occurs in flames, or discharge lamps. Stimulated emission occurs when matter in an excited state is perturbed by a photon of light and gives rise to a further photon of light, typically at the same energy and phase as the perturbing photon. This phenomenon is the process which gives rise to laser emission where you have many photons at the same wavelength and in phase with each other.
Black body radiation
A body at a given temperature also emits a characteristic spectrum of light called black body radiation. Consider an electric filament as current is applied to it. As the electric current supplies energy to the filament and it heats up, it starts to glow red, and as it gets hotter it then turns orange and then white. The process underlying this is well understood for a theoretical body known as a ‘black body’. Our filament will approximate a black body and as the filament gains energy from the electrical power it tries to equalize its energy with its surroundings by radiating its excess energy. It does this by emitting light starting first in the infrared and as the filament gets hotter or has more energy the radiation moves more into the visible spectrum.
The spectral radiance emittance M in Wm-2 nm-1 sr-1 of a black body of Temperature T in Kelvin is given by Planck’s Law below:
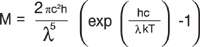
Where c is the speed of light, h= Planck’s constant and K = Boltzmann constant
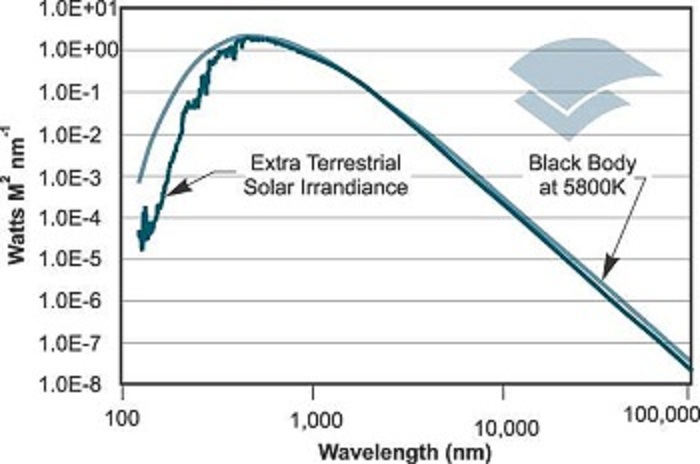
The spectral irradiance for artificial sources in general deviate from a perfect black body radiation, but the approximation is useful in many applications and by measuring the spectral output of a heated body, its temperature can be remotely measured. For example, Sunlight is due to the black body radiation characteristic of a body at approximately 5,800K (see graph below):
The source of the excitation to produce light emission can come from a variety of sources. The table below provides some of the sources and examples of where they can be used:
| Name | Excitation source | Examples of use |
| Chemiluminescence | Chemical reactions | Emergency lighting |
| Cathodeluminescence | Electron beam | Electron beam |
| Sonoluminescence | Sound energy | Possible chemical reactions |
| Triboluminescence | Friction energy | Gives rise to light emission seen when opening gum labels in the dark. |
| Bioluminescence | Biological processes | Light emission seen from Fireflies or some jellyfish |
| Thermoluminescence | Heat energy | Used for Archeological dating |
| Electroluminescence | Electric Voltage | Source of light seen in LED's |
| Photoluminescence | Photons of Light | Fluorescence markers |
Free charged particle acceleration
Light can also be produced by the acceleration of a free charged particle, such as an electron. The light emission is known as Bremsstrahlung or 'braking radiation'. The emission is characteristically seen in X-ray emission tubes which work by accelerating electrons with a high voltage and then by decelerating them very fast by directing them onto a metal target.
A special variety of particle accelerators known as Synchrotrons can be used to generate a wide range of light frequencies of very high power for use in the study of matter. A related effect is Cherenkov radiation which occurs when charged particles move through a medium faster than the speed of light. This produces the characteristic blue light seen in water ponds containing nuclear fuel.
Discover more about Light Emission in our Learning Centre.
